Anti-ACTA2: Mouse ACTA2 Antibody |
 |
BACKGROUND The six actin isoforms found in mammalians constitute a family of closely related proteins expressed in a tissue-specific way. β- and γ-cytoplasmic actins are ubiquitous, and four muscle actins with very similar primary sequences [alpha-skeletal (alpha-SKA), alpha-cardiac (alpha-CAA), alpha-smooth muscle (alpha-SMA) and γ-smooth muscle actin (γ-SMA)] are found in the different muscle types. Actin is a globular protein that is one of the most highly-conserved proteins known. It is found in two main states; G-actin is the globular monomeric form, whereas F-actin forms helical polymers. Both G-and F-actin are intrinsically flexible structures - a feature vital in actin's role as a dynamic filament network. Actin has four major functions. Firstly, F-actin polymers form microfilaments - polar intracellular 'tracks' for kinesin motor proteins, allowing the transport of vesicles, organelles and other cargo. Actin is a component of the cytoskeleton and links to alpha-actinin, E-cadherin and beta-catenin at adherens junctions. This gives mechanical support to cells and attaches them to each other and the extracellular matrix. In muscle cells, actin-rich thin filaments associate with myosin-rich thick filaments to form actomyosin myofibrils. Using energy from the hydrolysis of ATP, myofibrils undergo cyclic shortening through actin-myosin head interactions, which represents the mechanics of muscle contraction. Finally, actin has a role in cell motility through polymerization and depolymerization of fibrils.1
Alpha-smooth muscle actin (alpha-SMA, or ACTA2) is 42 kD actin isoform. alpha-SMA is transiently expressed in the myocardium and skeletal muscle during the development of the embryo and is highly restricted in adult animals to smooth muscle cells (SMCs); however, it is also expressed in myofibroblasts during wound healing and in a very limited number of adult tissues. These cells are present in healing wounds, scars, and fibrocontractive lesions where they contribute to fibrosis.2 Myofibroblast differentiation in vivo has been proposed to be dependent on a number of local environmental cues, including local production of growth factors such as transforming growth factor (TGF)-β1, extracellular matrix-integrin interactions, and mechanical stress. A number of cis-elements and trans-acting factors have been described that regulate gene expression of alpha-SMA in SMCs. Moreover, in myofibroblasts, cell-generated traction forces associated with alpha-SMA contribute to matrix remodeling, but exogenous mechanical forces can also increase alpha-SMA expression. Force-induced alpha-SMA utilizes a feed-forward amplification loop involving a priori alpha-SMA in focal adhesions, the binding of the p38 MAP kinase to alpha-SMA filaments, activation of Rho and binding of serum response factor to the CArG-B box of the alpha-SMA promoter. Thus, in addition to its importance as a structural protein in tissue remodeling and contraction, alpha-SMA may serve as a mechanotransducer, based on its ability to physically link mechanosensory elements and to enhance its own, force-induced expression.3
Alpha-smooth muscle actin (alpha-SMA, or ACTA2) is 42 kD actin isoform. alpha-SMA is transiently expressed in the myocardium and skeletal muscle during the development of the embryo and is highly restricted in adult animals to smooth muscle cells (SMCs); however, it is also expressed in myofibroblasts during wound healing and in a very limited number of adult tissues. These cells are present in healing wounds, scars, and fibrocontractive lesions where they contribute to fibrosis.2 Myofibroblast differentiation in vivo has been proposed to be dependent on a number of local environmental cues, including local production of growth factors such as transforming growth factor (TGF)-β1, extracellular matrix-integrin interactions, and mechanical stress. A number of cis-elements and trans-acting factors have been described that regulate gene expression of alpha-SMA in SMCs. Moreover, in myofibroblasts, cell-generated traction forces associated with alpha-SMA contribute to matrix remodeling, but exogenous mechanical forces can also increase alpha-SMA expression. Force-induced alpha-SMA utilizes a feed-forward amplification loop involving a priori alpha-SMA in focal adhesions, the binding of the p38 MAP kinase to alpha-SMA filaments, activation of Rho and binding of serum response factor to the CArG-B box of the alpha-SMA promoter. Thus, in addition to its importance as a structural protein in tissue remodeling and contraction, alpha-SMA may serve as a mechanotransducer, based on its ability to physically link mechanosensory elements and to enhance its own, force-induced expression.3
REFERENCES
1. Pollard, T.D. & Cooper, J.A.: Science 326:1208-12, 2009
2. Cherng, S. et al: J. Am. Sci. 4:7-9, 2008
3. Hinz, B. et al: Mol. Biol. Cell 14:2508-19, 2003
2. Cherng, S. et al: J. Am. Sci. 4:7-9, 2008
3. Hinz, B. et al: Mol. Biol. Cell 14:2508-19, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10399 |
Antigen: | A recombinant human alpha-SMA fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
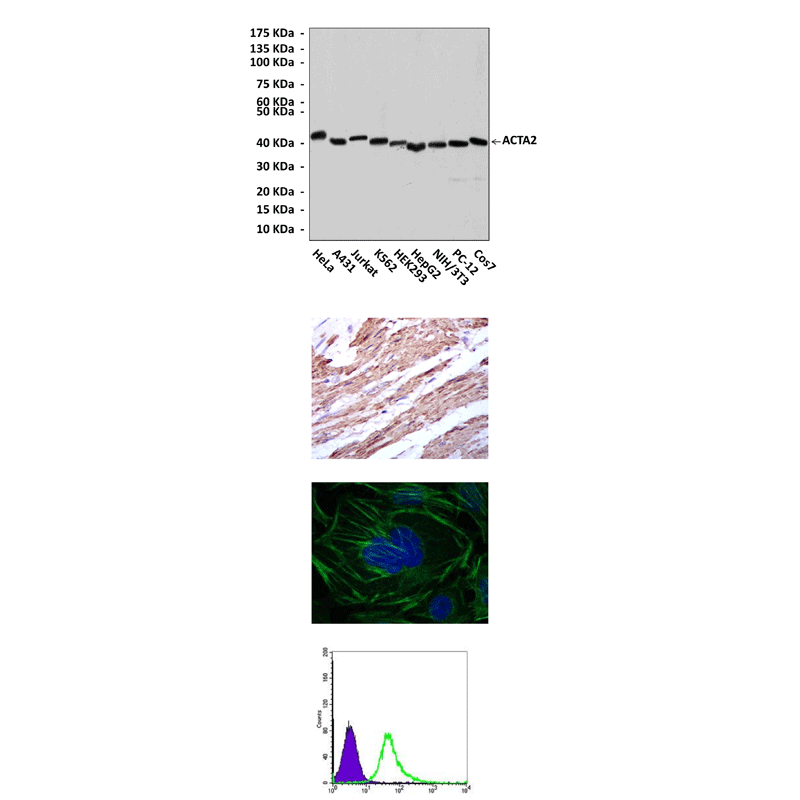
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 42 kDa |
Specificity/Sensitivity: | Detects alpha-SMA proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой