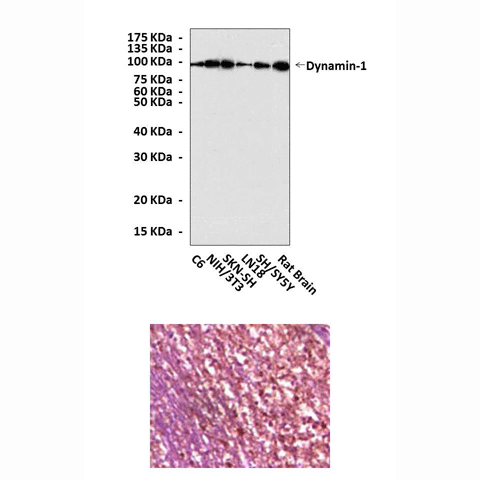
Anti-Dynamin-1: Mouse Dynamin-1 Antibody |
 |
BACKGROUND Dynamins are large GTPase superfamily that, in eukaryotic cells, includes classical Dynamins, Dynamin-like proteins, OPA1, Mx proteins, mitofusins and guanylate-binding proteins/atlastins.1 They are involved in many processes including budding of transport vesicles, division of organelles, cytokinesis and pathogen resistance. Mammalian classical dynamin occurs in at least three different isoforms, all of them with additional splicing alternatives at three different sites. The originally isolated Dynamin-1 is expressed exclusively in neurons, Dynamin-3 in the testes and Dynamin-2, which is 79% identical to both Dynamin-1 and shibire, is ubiquitously expressed. They are essential components of vesicle formation in receptor-mediated endocytosis, synaptic vesicle recycling, caveolae internalization, and possibly vesicle trafficking in and out of the Golgi. In addition to the GTPase domain, Dynamin also contains a pleckstrin homology domain (PH) implicated in membrane binding, a GTPase effector domain (GED) shown to be essential for self-assembly and stimulated GTPase activity, and a C-terminal proline-rich domain (PRD), which contains several SH3-binding sites. Dynamin partners bind to the PRD and may either stimulate Dynamin’s GTPase activity or target dynamin to the plasma membrane. Purified Dynamin readily self-assembles into rings or spirals. This striking structural property supports the hypothesis that Dynamin wraps around the necks of budding vesicles where it plays a key role in membrane fission.2
Dynamin-1 is rapidly dephosphorylated upon depolarization of nerve terminals. Dynamin-1 can be phosphorylated by protein kinase C (PKC) in vitro and this phosphorylation stimulates intrinsic dynamin GTPase activity.3 Although Dynamin-1 and Dynamin-2 seem to be identical in function, their regulation might be different as the process of synaptic vesicle recycling requires specific control of neuronal Dynamin. Amphiphysin, a SH3 domain containing neuronal protein, might be involved in synaptic vesicle endocytosis. It specifically interacts with Dynamin in vitro and colocalizes with Dynamin. Dynamin-2 association with SH3 domain-containing proteins might also be relevant for the regulation in nonneuronal cells.4
REFERENCES
1. Urrutia, R. et al: Proc. Natl. Acad. Sci. USA 94:377-84, 1997
2. Roux, A. et al: Nature 441:528-31, 2006
3. Robinson, P.J. et al: Nature 365:163-6, 1993
4. Solomaha,E. et al: J. Biol. Chem. 280:23147-56, 2005
2. Roux, A. et al: Nature 441:528-31, 2006
3. Robinson, P.J. et al: Nature 365:163-6, 1993
4. Solomaha,E. et al: J. Biol. Chem. 280:23147-56, 2005
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10073 |
Antigen: | Purified recombinant human Dynamin-1 fragments expressed in E. coli. |
Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 98 kDa |
Specificity/Sensitivity: | Detects endogenous Dynamin-1 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой