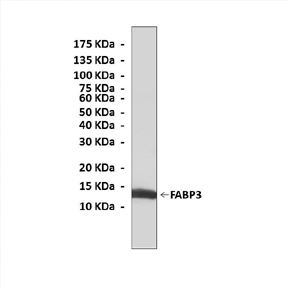
Anti-FABP3: Rabbit Fatty Acid-Binding Protein 3 Antibody |
 |
BACKGROUND Fatty acid-binding proteins (FABPs) are members of the superfamily of lipid-binding proteins (LBP). So far 9 different FABPs, with tissue-specific distribution, have been identified: L (liver), I (intestinal), H (muscle and heart), A (adipocyte), E (epidermal), Il (ileal), B (brain), M (myelin) and T (testis). The primary role of all the FABP family members is regulation of fatty acid uptake and intracellular transport. The structure of all FABPs is similar – the basic motif characterizing these proteins is beta-barrel, and a single ligand (e.g. a fatty acid, cholesterol, or retinoid) is bound in its internal water-filled cavity. Despite the wide variance in the protein sequence, the gene structure is identical. The FABP genes consist of 4 exons and 3 introns and a few of them are located in the same chromosomal region. For example, A-FABP, E-FABP and M-FABP create a gene cluster.1 Physiological roles of these proteins are not only involved in FA transport, but also in regulation of cell growth and differentiation, cellular signalling, gene transcription and cytoprotection.2 Because of their physiological properties some FABP genes were tested in order to identify mutations altering lipid metabolism and relating with other diseases.
Heart-type FABP3 (H-FABP), which is most abundantly expressed in heart, skeletal muscle, brain, and other tissues, is induced in brown adipose tissue (BAT) in hibernating squirrel and bat. It is also induced by acute cold exposure in rat. The physiological roles of FABP3 have been investigated with knockout mouse models. Characterization of FABP3-deficient mice has focused largely on effects on fatty acid metabolism in heart and skeletal muscle, issues in which this protein is abundantly expressed. In vivo labeling studies revealed reduced palmitic and arachidonic acid uptake in heart of Fabp3–/– mice (17), and reduced palmitic acid uptake and utilization in isolated cardiomyocytes. There was a compensatory reliance on glucose oxidation in the heart of Fabp3–/– mice. Fabp3–/– and Fabp3+/– mice also exhibit increased insulin sensitivity, perhaps related to the increased reliance on glucose rather than fatty acid fuels. Thus, FABP3 has important roles in fatty acid metabolism in heart and skeletal muscle, with effects on systemic glucose homeostasis. The induction of FABP3 in BAT during cold exposure described above raised the possibility that this protein may have a role in fatty acid trafficking in adaptive thermogenesis. Moreover, it was also shown that Fabp3+/– mice exhibit dopamine D2 receptor (D2R) dysfunction in the striatum. FABP3/H-FABP regulates functions of the dopamine D2R in the brain, through neuronal D2LR/FABP3 interaction.3 In addition, it was repoeted that FABP3/H-FABP inhibits growth of mammary epithelial cells. FABP3/H-FABP is a candidate tumor suppressor for human breast cancer. Furthermore, FABP3/H-FABP has been used as a serological biomarker of cardiac and skeletal muscle injury.4
Heart-type FABP3 (H-FABP), which is most abundantly expressed in heart, skeletal muscle, brain, and other tissues, is induced in brown adipose tissue (BAT) in hibernating squirrel and bat. It is also induced by acute cold exposure in rat. The physiological roles of FABP3 have been investigated with knockout mouse models. Characterization of FABP3-deficient mice has focused largely on effects on fatty acid metabolism in heart and skeletal muscle, issues in which this protein is abundantly expressed. In vivo labeling studies revealed reduced palmitic and arachidonic acid uptake in heart of Fabp3–/– mice (17), and reduced palmitic acid uptake and utilization in isolated cardiomyocytes. There was a compensatory reliance on glucose oxidation in the heart of Fabp3–/– mice. Fabp3–/– and Fabp3+/– mice also exhibit increased insulin sensitivity, perhaps related to the increased reliance on glucose rather than fatty acid fuels. Thus, FABP3 has important roles in fatty acid metabolism in heart and skeletal muscle, with effects on systemic glucose homeostasis. The induction of FABP3 in BAT during cold exposure described above raised the possibility that this protein may have a role in fatty acid trafficking in adaptive thermogenesis. Moreover, it was also shown that Fabp3+/– mice exhibit dopamine D2 receptor (D2R) dysfunction in the striatum. FABP3/H-FABP regulates functions of the dopamine D2R in the brain, through neuronal D2LR/FABP3 interaction.3 In addition, it was repoeted that FABP3/H-FABP inhibits growth of mammary epithelial cells. FABP3/H-FABP is a candidate tumor suppressor for human breast cancer. Furthermore, FABP3/H-FABP has been used as a serological biomarker of cardiac and skeletal muscle injury.4
REFERENCES
1. Merkel, M. Et al: J. Biol. Chem. 277:7405-11, 2001
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Shioda, N. et al: J. Neurosci. 30:3146-55, 2010
4. Pritt, M.L. et al: Toxicol. Sci. 103:382-96, 2008
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Shioda, N. et al: J. Neurosci. 30:3146-55, 2010
4. Pritt, M.L. et al: Toxicol. Sci. 103:382-96, 2008
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1336 |
Antigen: | Short peptide from human FABP3 sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 14 kDa |
Specificity/Sensitivity: | Detects endogenous levels of FABP3 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой