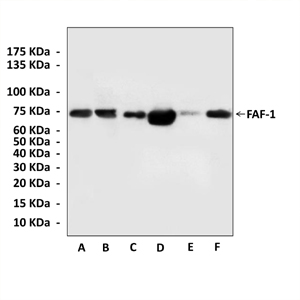
Anti-FAF-1: Rabbit Fas-Associated Factor 1 Antibody |
 |
BACKGROUND Fas-associated factor (FAF)-1 is a 74-kDa novel protein that has multiubiquitin-related domains, which was initially identified as a Fas-associated factor in mice. FAF1 protein can be broken up into several domains based on sites of protein interactions. The N-terminus contains the FID (Fas-interacting domain) from amino acids 1 – 181. UB1 (ubiquitin-homologous domain 1) spans amino acids 110 – 169 within the FID of FAF1. The DEDID (death effector domain-interacting domain) spans amino acids 181 – 381 and contains two phosphorylation sites located at Ser 289 and Ser 291. UB2 (ubiquitin homologous domain 2) spans amino acids 205 – 260 within the DEDID of FAF1. UAS (ubiquitin associated domain) spans amino acids 336 – 386 within the DEDID through to the C-terminus of FAF1. UBX (ubiquitin-like regulatory X domain) spans amino acids 567 – 650 in the C-terminus of FAF1. FAF1 is evolutionarily conserved, and in humans it is expressed abundantly in the testes, and slightly less in skeletal muscle and the heart. It is also highly expressed in the developing telencephalon with dynamic expression patterns at different embryonic stages, although it progressively becomes confined within limited regions. FAF-1 has been shown to play an important role in normal development and neuronal cell survival, whereas FAF1 down regulation may contribute to multiple aspects of tumorigenesis.1
FAF-1 acts as a suppressor of NF-kappa-B activity by interfering with nuclear translocation of the RelA subunit of NF-kappa-B. FAF1 is reported to be an inhibitor of I-kappa-B kinase activation too.2 The phosphorylated FAF-1 is reported to mediate the ubiquitin-independent, proteasome-dependent degradation of Aurora-A, and most recently as a key component of the TNF-alpha/NF-kappa-B signaling node that has been independently implicated in asbestos-induced oncogenesis through Arf inactivation. In addition to being a member of the Fas death-inducing signaling complex, FAF-1 reportedly interacts with a number of other proteins.3 For example, the N-terminal ubiquitin-associated domain of human FAF-1 (hFAF1-UBA) is associated with polyubiquitinated proteins in a linkage-specific manner and not to monoubiquitin, whereas the UBL1 (ubiquitin-like protein) binds tightly to the N-terminal region of the 70-kDa heat shock protein family, Hsp70/Hsc70, regardless of ATP and heat shock treatment. In addition, the C-terminal UBX domain binds the 97-kDa valosin-containing protein, a mutiubiquitin chain-targeting factor used in degradation via the ubiquitin-proteasome pathway. These findings suggest that FAF-1 may serve as a scaffolding protein that regulates protein degradation in the ubiquitin-proteasome pathway. A smaller 40-kDa FAF-1 protein has also been detected in certain tissues, although it is not yet clear if the smaller form has alternate functions. FAF1 is modified post-translationally via phosphorylation at potential phosphorylation sites Y225, S270, S289, S291 and S320. The protein kinase Casein Kinase 2 (CK2) has been shown to phosphorylate FAF-1 at S289 and S291 to potentially regulate cell cycle progression, apoptosis, and intracellular location, whereas Aurora A (Aur-A) phosphorylates these same residues via a feedback loop that involves FAF-1-mediated proteasome-dependent degradation of Aur-A.4
FAF-1 acts as a suppressor of NF-kappa-B activity by interfering with nuclear translocation of the RelA subunit of NF-kappa-B. FAF1 is reported to be an inhibitor of I-kappa-B kinase activation too.2 The phosphorylated FAF-1 is reported to mediate the ubiquitin-independent, proteasome-dependent degradation of Aurora-A, and most recently as a key component of the TNF-alpha/NF-kappa-B signaling node that has been independently implicated in asbestos-induced oncogenesis through Arf inactivation. In addition to being a member of the Fas death-inducing signaling complex, FAF-1 reportedly interacts with a number of other proteins.3 For example, the N-terminal ubiquitin-associated domain of human FAF-1 (hFAF1-UBA) is associated with polyubiquitinated proteins in a linkage-specific manner and not to monoubiquitin, whereas the UBL1 (ubiquitin-like protein) binds tightly to the N-terminal region of the 70-kDa heat shock protein family, Hsp70/Hsc70, regardless of ATP and heat shock treatment. In addition, the C-terminal UBX domain binds the 97-kDa valosin-containing protein, a mutiubiquitin chain-targeting factor used in degradation via the ubiquitin-proteasome pathway. These findings suggest that FAF-1 may serve as a scaffolding protein that regulates protein degradation in the ubiquitin-proteasome pathway. A smaller 40-kDa FAF-1 protein has also been detected in certain tissues, although it is not yet clear if the smaller form has alternate functions. FAF1 is modified post-translationally via phosphorylation at potential phosphorylation sites Y225, S270, S289, S291 and S320. The protein kinase Casein Kinase 2 (CK2) has been shown to phosphorylate FAF-1 at S289 and S291 to potentially regulate cell cycle progression, apoptosis, and intracellular location, whereas Aurora A (Aur-A) phosphorylates these same residues via a feedback loop that involves FAF-1-mediated proteasome-dependent degradation of Aur-A.4
REFERENCES
1. Menges, C.W. et al: Cell cycle 8:2528-34, 2009
2. Park, M.Y. et al: J. Biol. Chem. 279:2544-9, 2004
3. Song, J. et al: Prot. Sci. 18:2265–76, 2009
4. Jang, M.S. et al: J. Biol. Chem. 283:32344-51, 2008
2. Park, M.Y. et al: J. Biol. Chem. 279:2544-9, 2004
3. Song, J. et al: Prot. Sci. 18:2265–76, 2009
4. Jang, M.S. et al: J. Biol. Chem. 283:32344-51, 2008
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1337 |
Antigen: | Short peptide from human FAF-1 sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 74 kDa |
Specificity/Sensitivity: | Detects endogenous levels of FAF-1 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой