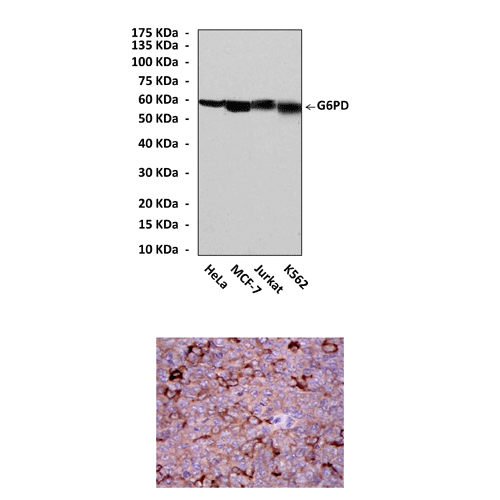
Anti-G6PD: Mouse G6PD Antibody |
 |
BACKGROUND The enzyme, glucose-6-phosphate dehydrogenase (G6PD or G6PDH, EC1.1.1.49), has long been considered and studied as the archetypical X-linked "housekeeping" enzyme that is present in all cells, where it plays the key role in regulating carbon flow through the pentose phosphate pathway. Specifically, the enzyme catalyzes the first reaction in the pathway leading to the production of pentose phosphates and reducing power in the form of NADPH for reductive biosynthesis and maintenance of the redox state of the cell. G6PD converts glucose-6-phosphate into 6-phosphoglucono-δ-lactone and is the rate-limiting enzyme of the pentose phosphate pathway. While the gene can be considered to be a constitutively expressed "housekeeping" gene in many tissues, there are several other tissues (liver, adipose, lung, and proliferating cells) wherein modulation of cellular G6PDH activity represents an important component of the integrated response to external stimuli (hormones, growth factors, nutrients, and oxidant stress). In this regard, adaptive regulation of G6PDH has been found to be exerted at transcriptional and posttranscriptional levels. G6PD is a typical cytoplasmic enzyme, although some G6PD activity is associated with peroxisomes in liver andkidney cells. The enzymatically active form of G6PD is either a dimer or a tetramer of a single polypeptide subunit of about 59 kd. Glucose-6-phosphate dehydrogenase is stimulated by its substrate Glucose 6 Phosphate. The usual ratio of NADPH/NADP+ in the cytosol of tissues engaged in biosyntheses is about 100/1. Increased utilization of NADPH for fatty acid biosynthesis will dramatically increase the level of NADP+, thus stimulating G6PD to produce more NADPH.1
G6PD is remarkable for its genetic diversity. Many variants of G6PD, mostly produced from missense mutations, have been described with wide ranging levels of enzyme activity and associated clinical symptoms. Two transcript variants encoding different isoforms have been found for this gene. Deficiency of G6PDH is a common genetic abnormality affecting millions of people worldwide. Many sequence variants, most caused by single point mutations, are known, exhibiting a wide variety of phenotypes. G6PD deficiency causes acute hemolytic anemia in the presence of simple infection, ingestion of fava beans, or reaction with certain medicines, antibiotics, antipyretics, and antimalarials.2
G6PD is remarkable for its genetic diversity. Many variants of G6PD, mostly produced from missense mutations, have been described with wide ranging levels of enzyme activity and associated clinical symptoms. Two transcript variants encoding different isoforms have been found for this gene. Deficiency of G6PDH is a common genetic abnormality affecting millions of people worldwide. Many sequence variants, most caused by single point mutations, are known, exhibiting a wide variety of phenotypes. G6PD deficiency causes acute hemolytic anemia in the presence of simple infection, ingestion of fava beans, or reaction with certain medicines, antibiotics, antipyretics, and antimalarials.2
REFERENCES
1. Kletzien,R.F. et al: FASEB J. 8:174-81, 1994
2. Groneborn, A.M. et al: FEBS J. 145:365-71, 1984
2. Groneborn, A.M. et al: FEBS J. 145:365-71, 1984
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10428 |
Antigen: | Raised against recombinant human G6PD fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:50 IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 59 kDa |
Specificity/Sensitivity: | Detects endogenous G6PD proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой