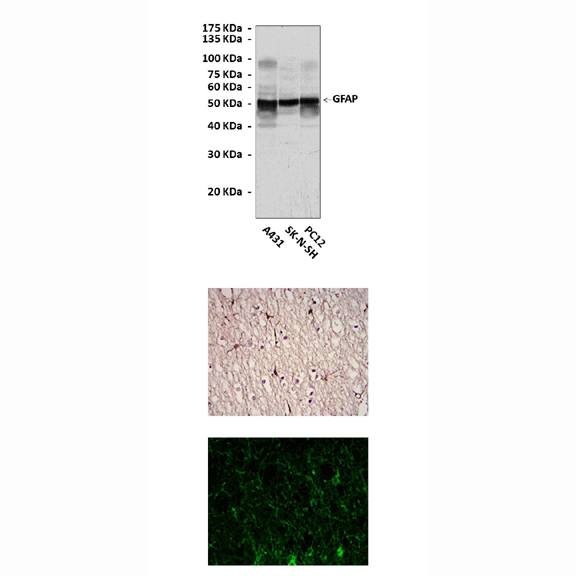
Anti-GFAP: Mouse Glial Fibrillary Acidic Protein Antibody |
 |
BACKGROUND Intermediate filament (IF) proteins constitute an extremely large multigene family of developmentally and tissue-regulated cytoskeleton proteins abundant in most vertebrate cell types. Glial fibrillary acidic protein (GFAP) is a 50-kD intracytoplasmic filamentous protein that constitutes a portion of, and is specific for, the cytoskeleton of the astrocyte. This protein has proved to be the most specific marker for cells of astrocytic origin under normal and pathological conditions. The two major IF proteins of astrocytes are vimentin and GFAP. Early during development, radial glia and immature astrocytes express mainly vimentin. Towards the end of gestation, a switch occurs whereby vimentin is progressively replaced by GFAP in differentiated astroglial cells. Thus, GFAP is recognized as an astrocyte maturation marker, constituting the major IF protein of mature astrocyte.1 Levels of GFAP are regulated under developmental and pathological conditions. Upregulation of GFAP expression is one of the main characteristics of the astrocytic reaction commonly observed after central nervous system (CNS) lesion. Interestingly, with increasing astrocytic malignancy, there is progressive loss of GFAP production. In addition, nestin, a currently used marker of neural stem cells, is transiently co-expressed with GFAP during development and is induced in reactive astrocytes following brain injury.2 In this way, studies on GFAP regulation have been shown to be useful to understand not only brain physiology but also neurological disease. Modulators of GFAP expression include several hormones such as thyroid hormone, glucocorticoids and several growth factors such as FGF, CNTF and TGF beta, among others. Studies of the GFAP gene have already identified several putative growth factor binding domains in its promoter region. Data obtained from transgenic and knockout mice have provided new insights into IF protein functions.3 More and more studies suggested an expanded role of GFAP in complex cellular events such as cytoskeletal reorganization, maintenance of myelination, cell adhesion, and signaling pathways. As such, GFAP may not represent a mere mechanical integrator of cellular space, as has been previously thought. Rather, GFAP may provide docking sites for important kinases that recognize key cellular substrates that enable GFAP to form a dynamic continuum with microfilaments, integrin receptors, and the extracellular matrix.4
REFERENCES
1. Eng, L.F. et al: Neurochem. Res. 25:1439-51, 2000
2. Bramanti, V. et al: Front. Biosci. (Schol Ed). 2:558-70, 2010
3. Ribotta, M.G. et al: Acta Neurochir. Suppl. 89:87-92, 2004
4. Rutka, J.T. et al: J. Neurosurg. 87:420-30, 1997
2. Bramanti, V. et al: Front. Biosci. (Schol Ed). 2:558-70, 2010
3. Ribotta, M.G. et al: Acta Neurochir. Suppl. 89:87-92, 2004
4. Rutka, J.T. et al: J. Neurosurg. 87:420-30, 1997
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10267 |
Antigen: | Purified recombinant human GFAP fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 50 kDa |
Specificity/Sensitivity: | Detects GFAP proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой