Anti-Heparanase: Rabbit Heparanase Antibody |
 |
BACKGROUND Heparan sulfate proteoglycans (HSPGs) are ubiquitous macromolecules associated with the cell surface and extracellular matrix (ECM) of a wide range of cells. The basic HSPG structure consists of a protein core to which several linear heparan sulfate (HS) chains are covalently O-linked. HS chains, unique in their ability to bind a multitude of proteins, ensure that a wide variety of bioactive molecules bind to the cell surface and ECM and thereby function in the control of diverse normal and pathological processes.1
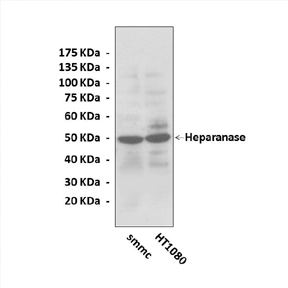
Heparanase is synthesized as a 65 kDa latent precursor that subsequently undergoes proteolytic processing by cathepsin L, yielding 8 kDa and 50 kDa protein subunits that undergo heterodimerization to form the active enzyme. Heparanase is an endo-glucuronidase that selectively cleaves extracellular and cell-surface heparan sulfate. It catalyzes the hydrolytic cleavage of the beta-1,4-glycosidic bond between a D-glucuronate and a D-glucosamine in heparan sulfate. The reaction mechanism probably involves general acid/base catalysis by Glu225 and nucleophilic catalysis by Glu343:Glu225 protonates the oxygen of the glycosidic bond, and Glu343 performs a nucleophilic attack on the anomeric carbon to break the glycosidic bond and form an unstable ester intermediate. The ester is hydrolyzed by an incoming water molecule and Glu225 now acts as a general base to activate the water and regain a proton.2
Heparanase has been identified in invasive normal and malignant cells, including activated cells of the immune system, cytotrophoblasts, keratinocytes, lymphoma, melanoma, myeloma and carcinoma cells. Heparan sulfate cleavage by heparanase could affect various biological processes. Heparanase plays important roles in cancer metastasis, angiogenesis and inflammation. It was suggested that a heparanase that cleaves heparin may be important in the degradation of the extracellular matrix by invading cells, notably metastatic malignant cells and migrating leukocytes to extravasate through the vascular basal lamina. Heparanase degraded both heparin and heparan sulfate to fragments of similar sizes.3 In addition, heparanase is involved in the regulation of the hemostatic system and it is directly involved in activation of the coagulation cascade.4
Heparanase is synthesized as a 65 kDa latent precursor that subsequently undergoes proteolytic processing by cathepsin L, yielding 8 kDa and 50 kDa protein subunits that undergo heterodimerization to form the active enzyme. Heparanase is an endo-glucuronidase that selectively cleaves extracellular and cell-surface heparan sulfate. It catalyzes the hydrolytic cleavage of the beta-1,4-glycosidic bond between a D-glucuronate and a D-glucosamine in heparan sulfate. The reaction mechanism probably involves general acid/base catalysis by Glu225 and nucleophilic catalysis by Glu343:Glu225 protonates the oxygen of the glycosidic bond, and Glu343 performs a nucleophilic attack on the anomeric carbon to break the glycosidic bond and form an unstable ester intermediate. The ester is hydrolyzed by an incoming water molecule and Glu225 now acts as a general base to activate the water and regain a proton.2
Heparanase has been identified in invasive normal and malignant cells, including activated cells of the immune system, cytotrophoblasts, keratinocytes, lymphoma, melanoma, myeloma and carcinoma cells. Heparan sulfate cleavage by heparanase could affect various biological processes. Heparanase plays important roles in cancer metastasis, angiogenesis and inflammation. It was suggested that a heparanase that cleaves heparin may be important in the degradation of the extracellular matrix by invading cells, notably metastatic malignant cells and migrating leukocytes to extravasate through the vascular basal lamina. Heparanase degraded both heparin and heparan sulfate to fragments of similar sizes.3 In addition, heparanase is involved in the regulation of the hemostatic system and it is directly involved in activation of the coagulation cascade.4
REFERENCES
1. Bernfield, M. et al: Annu. Rev. Biochem. 68:729–77, 1999
2. Gong, F. et al: J. Biol. Chem. 278, 35152-8, 2003
3. Ferro, V. et al: Mini. Rev. Med. Chem. 4: 693–702, 2004
4. Nadir, Y. et al: Haematologica 95:1927-34, 2010
2. Gong, F. et al: J. Biol. Chem. 278, 35152-8, 2003
3. Ferro, V. et al: Mini. Rev. Med. Chem. 4: 693–702, 2004
4. Nadir, Y. et al: Haematologica 95:1927-34, 2010
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1223 |
Antigen: | Short peptide from human heparanase sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 65, 50, 8 kDa |
Specificity/Sensitivity: | Detects endogenous levels of heparanase proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой