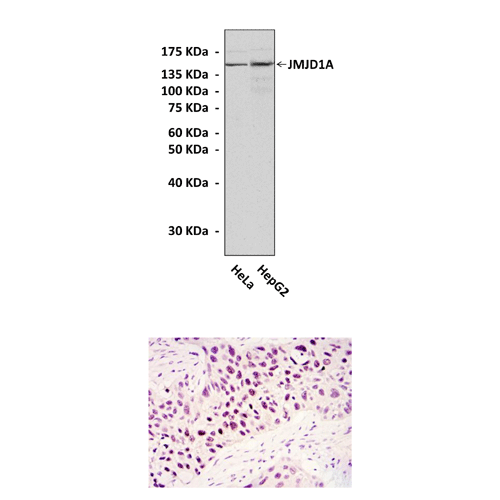
Anti-JMJD1A: Mouse JMJD1A/KDM3A/JHDM2A Antibody |
 |
BACKGROUND The methylation state of histones can be dynamically regulated by histone methyltransferases and demethylases. LSD1 (also known as AOF2) and the jumonji C (JmjC)-domain-containing proteins have been shown to possess histone demethylase activity. LSD1 catalyses removal of H3K4me2/H3K4me1 through a flavin-adenine-dinucleotide-dependent oxidation reaction. In contrast, JmjC-domain-containing proteins remove methyl groups from histones through a hydroxylation reaction that requires ketoglutarate and Fe(II) as cofactors.1 JHDM2A (also known as JMJD1A and KDM3A) is a JmjC domain-containing protein, which specifically demethylates mono- and dimethyl-H3K9. Mutational studies indicate that a JmjC domain and a zinc finger present in JHDM2A are required for its enzymatic activity.2 It was shown that JHDM2A plays an important role in nuclear hormone receptor-mediated gene activation and male germ cell development.3 In addition, JHDM2A was involved in regulating fat metabolic gene expression in muscle and brown fat tissue, and the knockout mice exhibited obesity and hyperlipidemia.4
REFERENCES
1. Bartova E et al.: J. Histochem. Cytochem. 56:711-721, 2008.
2. Lan F et al.: Curr Opin Cell Biol. 20: 316–325, 2008.
3. Yamane K et al.: Cell 125:483-495, 2006.
4. Tateishi K et al.: Nature 458:757-761, 2009.
2. Lan F et al.: Curr Opin Cell Biol. 20: 316–325, 2008.
3. Yamane K et al.: Cell 125:483-495, 2006.
4. Tateishi K et al.: Nature 458:757-761, 2009.
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10349 |
Antigen: | Raised against recombinant human JMJD1A fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mosue, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 150 kDa |
Specificity/Sensitivity: | Detects endogenous JMJD1A proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой