Anti-KCNN4: Polyclonal Calcium-Activated potassium channel Protein 1, Kca3.1, Antibody |
 |
BACKGROUND KCNN4 (also known as KCa3.1) is a calcium-activated intermediate-conductance potassium ion channel. It binds calmodulin tightly near the C-terminus, which serves as the Ca2+ sensor. KCNN4 channels are thus opened by a rise in cytosolic free Ca2+[Ca2+]i due to Ca2+-calmodulin-mediated cross-linking of subunits in the channel tetramer. In humans the channel is expressed in several secretory organs and subtypes of hematopoietic cells, but not detected in excitable tissues. The mRNA level for KCNN4 is upregulated in activated leukocytes, mitogen-induced endothelial cells and vascular smooth muscle cells, and several types of human cancers, suggesting a possible role for the channel in inflammatory and oncology diseases.1 Physiologically, KCNN4 has been demonstrated to play a role in acetylcholine and endothelium-derived hyperpolarizing factor (EDHF) induced hyperpolarization, and thus control of blood pressure. Pathophysiologically, KCNN4 contributes to proliferation of T-cells, B-cells, fibroblasts, and vascular SMCs, as well as the migration of SMCs and macrophages and platelet coagulation.2
KCNN4 function is reported to be increased by membrane-associated protein kinase A through phosphorylation of either the channel protein itself or a closely associated accessory protein in oocytes and T84 cells. In CD4+ T cells, KCNN4 activity is increased by the nucleoside diphosphate kinase B, which phosphorylates KCNN4 on histidine 358. In contrast, histidine 358 is dephosphorylated by the mammalian protein histidine phosphatase, which directly binds to the KCNN4 protein and negatively regulates T cell Ca2+ flux by decreasing KCNN4 activity. KCNN4 modulation in T cells is thus one of the rare examples of histidine phosphorylation/dephosphorylation influencing a biological process in mammals.3 Several potent and selective KCNN4 blockers, including clotrimazole and its analogs TRAM-34 and ICA-17043, have been used to investigate the involvement of the channel in human disease. The compounds have been shown to suppress the proliferation of several cancer cells in vitro and the growth of the corresponding cancers in vivo, consistent with an oncologic indication. TRAM-34 also ameliorates symptoms in experimental autoimmune encephalomyelitis and several models of cardiovascular diseases, arguing for a role of the channel in inflammatory diseases.4
KCNN4 function is reported to be increased by membrane-associated protein kinase A through phosphorylation of either the channel protein itself or a closely associated accessory protein in oocytes and T84 cells. In CD4+ T cells, KCNN4 activity is increased by the nucleoside diphosphate kinase B, which phosphorylates KCNN4 on histidine 358. In contrast, histidine 358 is dephosphorylated by the mammalian protein histidine phosphatase, which directly binds to the KCNN4 protein and negatively regulates T cell Ca2+ flux by decreasing KCNN4 activity. KCNN4 modulation in T cells is thus one of the rare examples of histidine phosphorylation/dephosphorylation influencing a biological process in mammals.3 Several potent and selective KCNN4 blockers, including clotrimazole and its analogs TRAM-34 and ICA-17043, have been used to investigate the involvement of the channel in human disease. The compounds have been shown to suppress the proliferation of several cancer cells in vitro and the growth of the corresponding cancers in vivo, consistent with an oncologic indication. TRAM-34 also ameliorates symptoms in experimental autoimmune encephalomyelitis and several models of cardiovascular diseases, arguing for a role of the channel in inflammatory diseases.4
REFERENCES
1. Chou, C.C. et al: Expert Rev Mol Diagn. 8:179-87, 2008
2. Tharp, D.L. & Bowles, D.K.: Cardiovasc Hematol Agents Med Chem. 7:1-11, 2009
3. Srivastava, S. et al: Mol. Cell 24:665-75, 2006
4. Bradding, P. & Wulff, H.: Br J Pharmacol. 157:1330–9, 2009
2. Tharp, D.L. & Bowles, D.K.: Cardiovasc Hematol Agents Med Chem. 7:1-11, 2009
3. Srivastava, S. et al: Mol. Cell 24:665-75, 2006
4. Bradding, P. & Wulff, H.: Br J Pharmacol. 157:1330–9, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
|
Cat.No.:
|
CA1788
|
|
Antigen:
|
N- terminal sequence of human KCNN4
|
|
Isotype:
|
Affinity-Purified Rabbit Polyclonal IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human, Rabbit, Rat, Mouse
|
|
Applications &
Suggested starting
dilutions:
|
WB 1:500 to 1:1000
IP n/d
IHC (Paraffin) 1:50 to 1:200 ICC n/d
FACS n/d
|
|
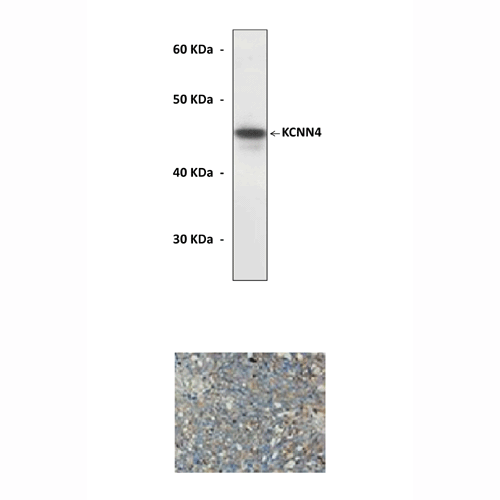
Predicted Molecular
Weight of protein:
|
46 kDa
|
|
Specificity/Sensitivity:
|
Reacts specifically with KCNN4 of human, rabbit, mouse & rat origin in immunostaining and western blotting, no cross-reactivity with other members of the family.
|
|
Storage:
|
Store at 4° C for frequent use; at -20° C for at least one year.
|
*Optimal working dilutions must be determined by end user.
|
| ||||||||||
| ||||||||||
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой