Anti-LMP7/PSMB8: Mouse LMP7/PSMB8 Antibody |
 |
BACKGROUND The ubiquitin-proteasome system (UPS) is among others involved in the regulation of protein quality control in the cells. The UPS with the 26S proteasome as central proteolytic unit represents the major ATP-dependent degradation system in eukaryotes responsible for the maintenance of protein homeostasis. Short-lived regulatory proteins involved in cell differentiation, cell-cycle regulation, transcriptional regulation, or apoptosis, but also aberrant proteins are directed to proteasomal degradation through conjugation with the small protein modifier ubiquitin via a cascade of E1, E2, and E3 enzymes, thus forming poly-ubiquitinylated (poly-ub) proteins. Poly-ub proteins are substrates for 26S proteasomes which are formed through the association of two 19S regulator complexes with the catalytic core complex, the 20S proteasome that hydrolyzes proteins into shorter peptide fragments. Peptide hydrolyzing activity of the 20S core is restricted to three β-subunits, i.e. β1, β2, and β5, located in the two inner heptameric β-rings of the 20S proteasome. In infectious disease, cells activated by interferons (IFNs) express three unique catalytic subunits β1i/LMP2, β2i/MECL-1 and β5i/LMP7 (PSMB8) forming an alternative proteasome isoform, the immunoproteasome (IP).1 IP-mediated proteolysis is responsible for the generation of immunogenic epitopes presented by MHC class I molecules, which activate antigen-specific CD8+ T cells.2 Upon interferon (IFN)-exposure of cells or tissues, three alternative catalytically active β subunits are induced. These so called immunosubunits are incorporated into newly formed 20S immunoproteasomes (IP) in a process that is driven by β5i/LMP7. β1i/LMP2 and β5i/LMP7 are encoded within the major histocompatibility II region and their incorporation into IPs induces altered proteolytic characteristics that result in many cases in more efficient liberation of MHC class I epitopes particularly within the early phase of antiviral immunity. This increase in MHC class I peptide supply by IPs appears to be important for triggering an effective early CD8 T cell response. However, an alternative physiological function of IPs has been demonstrated recently in that IPs protect cells against cytokine induced oxidative damage, thus preserving protein homeostasis. Substrate modification of oxidant-damaged proteins with poly-ubiquitin results in protein degradation particularly by IPs. Thus, a major innate function of IPs in viral infection is to stabilize cell viability in inflammatory tissue injury and prevent excessive inflammatory tissue damage in viral disease via preservation of protein homeostasis due to accelerated substrate turnover by IPs.3
REFERENCES
1. Fehling, H.J. et al: Science 265:1234-7, 1994
2. Groettrup, M. et al: J. Biol. Chem. 270:23808-15, 1995
3. Opitz, E. et al: PLoS Pathog 7: e1002233, 2011
2. Groettrup, M. et al: J. Biol. Chem. 270:23808-15, 1995
3. Opitz, E. et al: PLoS Pathog 7: e1002233, 2011
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10407 |
Antigen: | Raised against recombinant human LMP7 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human |
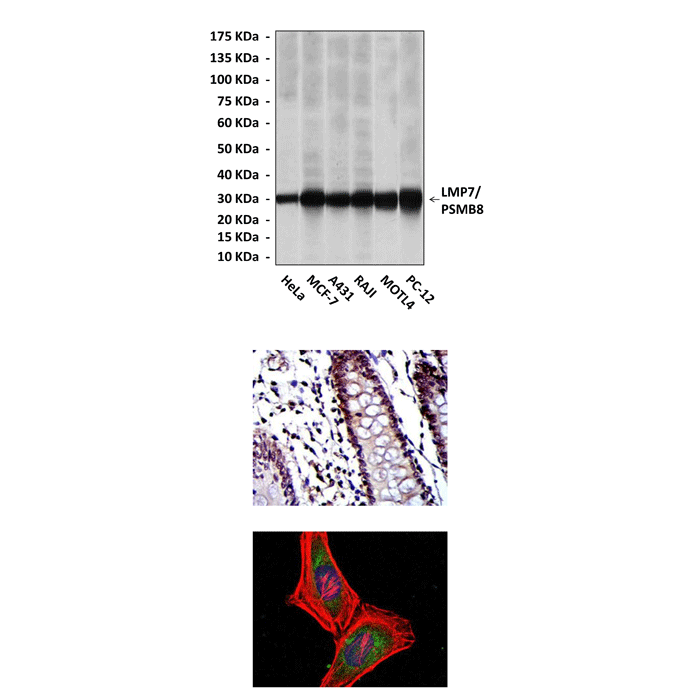
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 30 kDa |
Specificity/Sensitivity: | Detects LMP7 proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой