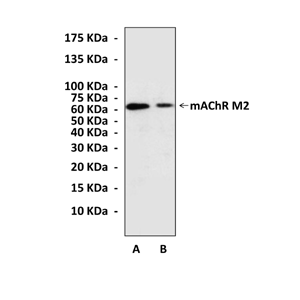
Anti-mAChR M2: Rabbit mAChR M2 (CHRM2) Antibody |
 |
BACKGROUND The muscarinic acetylcholine (ACh) receptors (mAChRs) were among the earliest members of the seven-transmembrane (TM) G-protein-coupled receptors to be defined pharmacologically. They play key roles in regulating the activity of many important functions of the central and peripheral nervous system.The five genetically distinct mAChR subtypes fall into two main groups. The M1, M3 and M5 mAChRs couple preferentially to G-proteins of the Gq/G11 class, which leads to phosphoinositide breakdown. In contrast, M2 and M4 mAChRs couple primarily to G-proteins of the Gi and Go classes, typically leading to adenylate cyclase inhibition and the activation of inward-rectifier potassium conductances.1 Among a plethora of other possible responses, in a suitable cellular context, all of the mAChR subtypes can activate non-receptor tyrosine kinases, transactivate the epidermal growth factor receptor and activate extracellular signal related protein kinase cascades.2
The M2 mAChR is responsible for various physiologically important regulatory functions in tissues such as heart, smooth muscle, and brain. M2 mAChRs are the primary muscarinic subtype in the heart where their stimulation leads to the regulation of myocardial contractility. As with other GPCRs, M2 mAChR activity is tightly regulated by desensitization and internalization. These regulatory mechanisms are typically associated with receptor phosphorylation followed by either recycling or down-regulation. It has been demonstrated that the agonist-dependent internalization of the M2mAChR by the endogenous machinery in HEK293 cells is independent of arrestins and dynamin. Agonist-dependent phosphorylation of the M2 mAChR does appear to play a key role in the internalization of these receptors, as phosphorylation-deficient mutants exhibit a reduction in the rate and extent of internalization. Although the M2 and M4 receptors transduce their signals through the same second messengers but internalize though different pathways. It was demonstrated that the M2 endocytic pathway utilizes Arf6 and Rab22 while M4 uses Rab5 and Rab11 for its endocytic pathway. Moreover, M2 and M4 do not colocalize in vesicles during their initial stages of endocytosis.3 In addition, it was also shown that allosteric modulators change the trafficking properties of M2 mAChRs by a mechanism that may involve a modification in receptor internalization properties. These changes in receptor trafficking can be manifested at both the level of ligand binding as well as the level of cellular responsiveness.4
The M2 mAChR is responsible for various physiologically important regulatory functions in tissues such as heart, smooth muscle, and brain. M2 mAChRs are the primary muscarinic subtype in the heart where their stimulation leads to the regulation of myocardial contractility. As with other GPCRs, M2 mAChR activity is tightly regulated by desensitization and internalization. These regulatory mechanisms are typically associated with receptor phosphorylation followed by either recycling or down-regulation. It has been demonstrated that the agonist-dependent internalization of the M2mAChR by the endogenous machinery in HEK293 cells is independent of arrestins and dynamin. Agonist-dependent phosphorylation of the M2 mAChR does appear to play a key role in the internalization of these receptors, as phosphorylation-deficient mutants exhibit a reduction in the rate and extent of internalization. Although the M2 and M4 receptors transduce their signals through the same second messengers but internalize though different pathways. It was demonstrated that the M2 endocytic pathway utilizes Arf6 and Rab22 while M4 uses Rab5 and Rab11 for its endocytic pathway. Moreover, M2 and M4 do not colocalize in vesicles during their initial stages of endocytosis.3 In addition, it was also shown that allosteric modulators change the trafficking properties of M2 mAChRs by a mechanism that may involve a modification in receptor internalization properties. These changes in receptor trafficking can be manifested at both the level of ligand binding as well as the level of cellular responsiveness.4
REFERENCES
1. Jones, K.T. et al: J. Mol. Signal. 1:7, 2006
2. Nathanson, N.M.:Proc. Natl. Acad. Sci. U.S.A. 97:6245–7, 2000
3. Reiner, C. & Nathanson, N.M.: Life Sci. 82:718-27, 2008
4. May, L.T. et al:J. Pharmacol. Exp. Therap. 312:382-90, 2005
2. Nathanson, N.M.:Proc. Natl. Acad. Sci. U.S.A. 97:6245–7, 2000
3. Reiner, C. & Nathanson, N.M.: Life Sci. 82:718-27, 2008
4. May, L.T. et al:J. Pharmacol. Exp. Therap. 312:382-90, 2005
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1325 |
Antigen: | Short peptide from human mAChR M2 sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 60-80 kDa |
Specificity/Sensitivity: | Detects endogenous levels of mAChR M2 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой