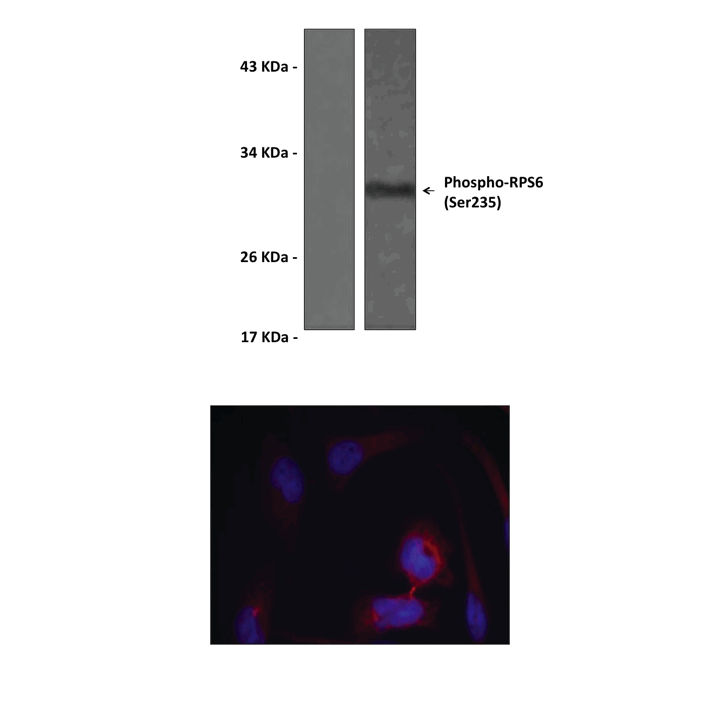
Anti-Phospho-RPS6: Rabbit Ribosomal S6 Protein, Phospho-Ser235/Ser236 Antibody |
 |
BACKGROUND Ribosomes, the organelles that catalyze protein synthesis, consist of a small 40S subunit and a large 60S subunit. Together these subunits are composed of 4 RNA species and approximately 80 structurally distinct proteins. Ribosomal protein S6 is a cytoplasmic ribosomal protein that is a component of the 40S subunit. It belongs to the S6E family of ribosomal proteins and is the major substrate of protein kinases in the ribosome, with subsets of five C-terminal serine residues phosphorylated by different protein kinases. Phosphorylation is induced by a wide range of stimuli, including growth factors, tumor-promoting agents, and mitogens. Dephosphorylation occurs at growth arrest. The ribosomal protein S6 may contribute to the control of cell growth and proliferation through the selective translation of particular classes of mRNA. As is typical for genes encoding ribosomal proteins, there are multiple processed pseudogenes of this gene dispersed through the genome.1
The Ser235/236 of ribosomal protein S6 was phosphorylated by p70S6K which was activated by mTOR/p70S6K signaling pathway. This signaling pathway mediates various biological effects of nutrients, insulin, and energy. Nutrients such as amino acids and glucose increase hVps34 activity, stimulating the production of PI3P. PI3P recruits proteins containing FYVE or PX domains to endosomal membranes. The mTOR/S6K signaling pathway is also activated by insulin. Insulin binds the insulin receptor and leads to an increase in tyrosine phosphorylation of IRS1. IRS1-associated class 1 PI3K increases the production of PIP3, recruiting PDK1 and PKB to the plasma membrane. PKB is activated by the concerted action of the rapamycin-insensitive rictor-mTOR complex and PDK1. Activated PKB phosphorylates and subsequently inactivates TSC2, a GTPase-activating protein, leading to an increase in GTP bound Rheb. Rheb-GTP increases mTOR activity and further facilitates the phosphorylation of p70S6K and 4E-BP1. The mTOR/p70S6K signaling pathway can sense energy levels either directly, through mTOR, or indirectly, through AMPK. Energy stress, such as fasting or lowering cellular ATP concentration, induces AMPK activation, leading to a PKB-independent TSC2 phosphorylation, which is thought to activate TSC2. This activation of TSC2 results in an increase in GDP bound Rheb and subsequent decrease in mTOR activity. Hypoxia induces Redd1/2, leading to a decrease of mTOR activity in a pathway parallel to the AMPK. Thus it is presumably mTOR/Raptor complex 1 that mediates p70S6K phosphorylation at a number of residues. These phosphorylation sites include T389, whose phosphorylation allows PDK1 to phosphorylate p70S6K T229 and activate the kinase. Moreover, the role of the mTOR/Rictor/G-beta-L complex (mTOR complex 2) in Akt activation places it as a key upstream effector in p70S6K activation.2 Once p70S6K is activated, it acts on downstream a number of effectors including 40S ribosomal protein S6 , protein synthesis initiation factor 4B, and elongation factor 2 kinase. Ribosomal protein S6 has been reported to be phosphorylated in an ordered fashion: Ser236->235->240->244->247. Hence, failure to detect phosphorylation of ribosomal protein S6 at Ser235/236 indicates that the protein is unphosphorylated.3 Ribosomal protein S6 phosphorylation following p70S6K activation increases the affinity of ribosomes for TOP mRNAs and thus facilitates their translation initiation. These particular mRNA transcripts (5'TOP) contain an oligopyrimidine tract in their 5' untranslated regions and encode components of the translational machinery and some proteins involved in cell cycle progression.4
The Ser235/236 of ribosomal protein S6 was phosphorylated by p70S6K which was activated by mTOR/p70S6K signaling pathway. This signaling pathway mediates various biological effects of nutrients, insulin, and energy. Nutrients such as amino acids and glucose increase hVps34 activity, stimulating the production of PI3P. PI3P recruits proteins containing FYVE or PX domains to endosomal membranes. The mTOR/S6K signaling pathway is also activated by insulin. Insulin binds the insulin receptor and leads to an increase in tyrosine phosphorylation of IRS1. IRS1-associated class 1 PI3K increases the production of PIP3, recruiting PDK1 and PKB to the plasma membrane. PKB is activated by the concerted action of the rapamycin-insensitive rictor-mTOR complex and PDK1. Activated PKB phosphorylates and subsequently inactivates TSC2, a GTPase-activating protein, leading to an increase in GTP bound Rheb. Rheb-GTP increases mTOR activity and further facilitates the phosphorylation of p70S6K and 4E-BP1. The mTOR/p70S6K signaling pathway can sense energy levels either directly, through mTOR, or indirectly, through AMPK. Energy stress, such as fasting or lowering cellular ATP concentration, induces AMPK activation, leading to a PKB-independent TSC2 phosphorylation, which is thought to activate TSC2. This activation of TSC2 results in an increase in GDP bound Rheb and subsequent decrease in mTOR activity. Hypoxia induces Redd1/2, leading to a decrease of mTOR activity in a pathway parallel to the AMPK. Thus it is presumably mTOR/Raptor complex 1 that mediates p70S6K phosphorylation at a number of residues. These phosphorylation sites include T389, whose phosphorylation allows PDK1 to phosphorylate p70S6K T229 and activate the kinase. Moreover, the role of the mTOR/Rictor/G-beta-L complex (mTOR complex 2) in Akt activation places it as a key upstream effector in p70S6K activation.2 Once p70S6K is activated, it acts on downstream a number of effectors including 40S ribosomal protein S6 , protein synthesis initiation factor 4B, and elongation factor 2 kinase. Ribosomal protein S6 has been reported to be phosphorylated in an ordered fashion: Ser236->235->240->244->247. Hence, failure to detect phosphorylation of ribosomal protein S6 at Ser235/236 indicates that the protein is unphosphorylated.3 Ribosomal protein S6 phosphorylation following p70S6K activation increases the affinity of ribosomes for TOP mRNAs and thus facilitates their translation initiation. These particular mRNA transcripts (5'TOP) contain an oligopyrimidine tract in their 5' untranslated regions and encode components of the translational machinery and some proteins involved in cell cycle progression.4
REFERENCES
1. Dufner, A. & Thomas, G.: Exp. Cell Res. 253:100-9, 1999
2. Um, S.H. et al: Cell Metab.3:393-402, 2006
3. Ferrar, S. et al: J. Biol. Chem. 266:22770-5, 2001
4. Jefferies, H. B. J. et al: Proc. Natl. Acad. Sci. USA 91:4441-5, 1994
2. Um, S.H. et al: Cell Metab.3:393-402, 2006
3. Ferrar, S. et al: J. Biol. Chem. 266:22770-5, 2001
4. Jefferies, H. B. J. et al: Proc. Natl. Acad. Sci. USA 91:4441-5, 1994
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1522 |
Antigen: | Peptide sequence around phosphorylation site of serine 235 (R-L-S(p)-S-L), according to the protein RPS6. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat, Mouse |
Applications & Suggested starting dilutions:* | WB 1:500-1:1000 IP n/d IHC n/d ICC n/d FACS n/d IF 1:100-1:200 |
Predicted Molecular Weight of protein: | 32 kDa |
Specificity/Sensitivity: | Detects endogenous Phospho-RPS6 (Ser235/Ser236) without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой