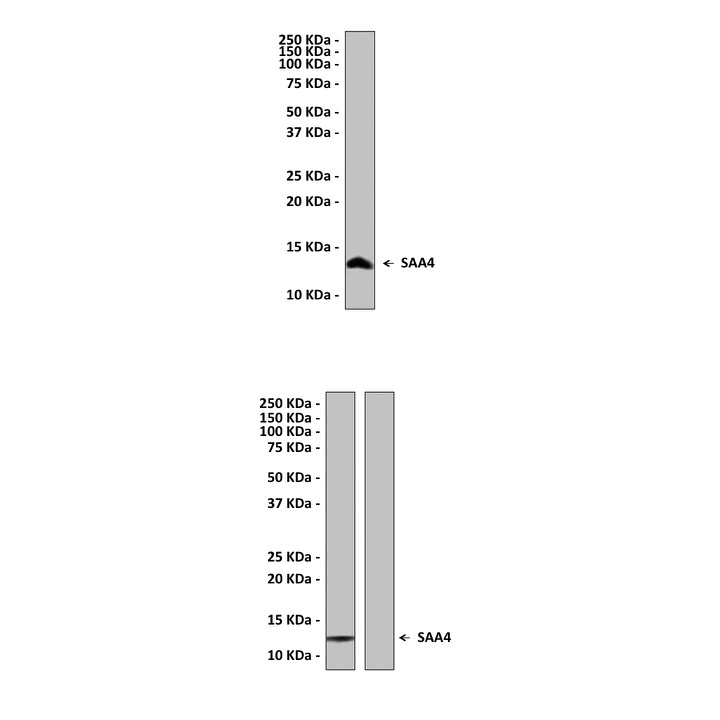
Anti-SAA4: Rabbit Serum Amyloid A4 Antibody |
 |
BACKGROUND Serum Amyloid A proteins (SAAs), a family of homologous molecules, are all apolipoproteins of HDL. They can be divided into two groups. The first group includes the well-characterized classical acute phase SAAs that increase dramatically during an acute phase response due to cytokine-driven hepatic synthesis. They displace apolipoprotein A-I (apoA-I) with resultant remodeling of HDL, yielding larger particles with a higher hydrated density, even becoming the major apolipoprotein component of acute phase HDL. The second group comprises the recently discovered constitutively expressed SAAs, namely mouse SAA5 and human SAA4. In humans, four SAA genes are located on chromosome 11. The acute-phase SAA proteins (A-SAAs) are encoded by two genes, SAA1 and SAA2, and allelic variations at these two loci account for the corresponding A-SAA1 and A-SAA2 isoforms. A-SAAs (12 kDa, 104 amino acids) are major acute-phase reactants whose in vivo concentrations increase by as much as 1000-fold during inflammation, thus representing ideal markers for clinical practice. Further, A-SAAs are precursor proteins of secondary reactive amyloidosis and are major apolipoproteins of high-density lipoproteins during inflammation. While the locus designated SAA3 is a pseudogene, the SAA4 locus encodes for a premolecule of 130 amino acids from which a signal peptide containing 18 residues is cleaved to yield 112 amino acid mature SAA4 protein. The SAA4 proteins of human and mouse have been found to be structurally similar and based on expression characteristics, sequences and positions within the human and mouse gene cluster the constitutively expressed SAA4 (also named C-SAA) is considered to be evolutionary homologues but behave like an outgroup in the SAA superfamily.1
Human SAA4 is a minor apolipoprotein component of lipoproteins of the high-density range constituting 1–2% of the total apolipoproteins. The distribution of SAA4 is restricted to two lipoprotein subclasses and therefore, SAA4 merits consideration as a factor involved in lipid transfer between lipoprotein classes. The serum concentration of SAA4 is 10-fold higher than that of A-SAA in the normal state but is not changed dramatically during the inflammatory state.2 Although lacking cytokine-responsive elements in the promoter region, cytokine- and glucocorticoid-mediated induction of human SAA4 mRNA in smooth muscle cells and monocytes/macrophages has been reported. Further credence for extrahepatic expression of SAA4 mRNA is derived from studies in human lesion material. This raises the possibility of similar proatherogenic properties of human SAA4 as reported for A-SAA. Other studies suggested that SAA4 is a minor acute phase reactant in humans. SAA4 concentrations showed a good agreement with serum pseudocholinesterase activity in healthy subjects and patients with lowered pseudocholinesterase when patients with elevated acute phase SAA were excluded. These results suggest that SAA4 can be an indicator of nutrition or of hepatic protein synthesis in the absence of inflammation.3
Human SAA4 is a minor apolipoprotein component of lipoproteins of the high-density range constituting 1–2% of the total apolipoproteins. The distribution of SAA4 is restricted to two lipoprotein subclasses and therefore, SAA4 merits consideration as a factor involved in lipid transfer between lipoprotein classes. The serum concentration of SAA4 is 10-fold higher than that of A-SAA in the normal state but is not changed dramatically during the inflammatory state.2 Although lacking cytokine-responsive elements in the promoter region, cytokine- and glucocorticoid-mediated induction of human SAA4 mRNA in smooth muscle cells and monocytes/macrophages has been reported. Further credence for extrahepatic expression of SAA4 mRNA is derived from studies in human lesion material. This raises the possibility of similar proatherogenic properties of human SAA4 as reported for A-SAA. Other studies suggested that SAA4 is a minor acute phase reactant in humans. SAA4 concentrations showed a good agreement with serum pseudocholinesterase activity in healthy subjects and patients with lowered pseudocholinesterase when patients with elevated acute phase SAA were excluded. These results suggest that SAA4 can be an indicator of nutrition or of hepatic protein synthesis in the absence of inflammation.3
REFERENCES
1. Hrzenjak,A. et al: Protein Eng. 14:949-52, 2001
2. de Beer, M.C. et al: J. Lipid Res. 36:526-34, 1995
3. Yamada, T. et al: Clin Chem Lab Med. 39:7-10, 2001
2. de Beer, M.C. et al: J. Lipid Res. 36:526-34, 1995
3. Yamada, T. et al: Clin Chem Lab Med. 39:7-10, 2001
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1538 |
Antigen: | SAA4 (NP_006503.1, 1 a.a. ~ 130 a.a) full-length human protein. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1-5 ug/mL IP n/d IHC n/d ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 14.8 kDa |
Specificity/Sensitivity: | Detects endogenous SAA4 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой