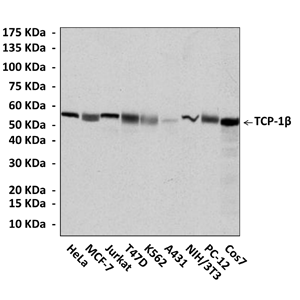
Anti-TCP-1β: Mouse TCP-1beta Antibody |
 |
BACKGROUND The family of molecular chaperones termed chaperonins can be categorised based on their sequences as either group I or group II chaperonins. The first includes the eubacterial chaperone GroEL and chaperonins from mitochondria and chloroplasts; the second includes the archaeal chaperonins and CCT, which is found in the cytoplasm of all eukaryotic cells and is essential in yeast. All chaperonins are large, barrel-shaped oligomers consisting of two rings of subunits stacked back-to-back surrounding a central cavity. Chaperonin containing TCP-1 (CCT) consists of eight distinct subunits, which are the products of individual genes. The eight CCT subunits, named α, β, γ, δ, ξ, ζ, η and θ (Cct1 to 8 in yeast), each occupy a fixed position in the chaperonin ring. Each consists of an equatorial domain that contains the ATP-binding site and both inter- and intra-ring contact sites, an apical, substrate-binding domain and an intermediate linker domain that relays nucleotide-induced conformational changes from the equatorial to the apical domains. These subunits display the most divergence in sequence in their apical, substrate-binding domains. Unlike GroEL, which is assisted by the lid-forming co-chaperone GroES, CCT has no such co-chaperone. Instead, a built-in lid is formed from helical protrusions that erupt from the apical domains of all eight CCT subunits and facilitate the encapsulation of folding substrates within the chaperonin cavity. The subunits thought to initiate ATP binding in one ring are in close proximity to those subunits at the end of this cascade in the other ring.1 However, Recent studies have established that the CCT function is regulated by phosducin-like proteins (PhLP) that are increasingly viewed as CCT co-chaperones.2
CCT participates in the folding of newly synthesized nascent polypeptides from ribosomes including actin, tubulin and several others. An increasing number of physiological CCT substrates have been identified; these include cyclin E, cdc20, pololike kinase 1 (PLK1), and Von Hippel-Lindau (VHL) tumor suppressor protein. Some data indicate that approximately 5-10% of newly synthesized proteins may flow through CCT. Many of these substrates are involved in cell cycle progression. Therefore, CCT plays an important role in cytoskeletal organization and cell division. Moreover, the protein folding activity of CCT might provide a mechanism to coordinate S6K- and RSK-regulated protein synthesis with protein folding. It was shown that RSK and S6K phosphorylate the CCTβ subunit at Ser-260. Furthermore, CCTβ plays an important role in regulating cell proliferation and Ser-260 phosphorylation contributes significantly to this process. Thus, the Ras-MAPK and PI3K-mTOR pathways utilize RSK and S6K to converge upon the phosphorylation and regulation of CCTβ function in mammalian cells.3
Recently, it was demonstrated that normal CCT function is ultimately required for the morphogenesis and survival of sensory neurons of the retina, and suggested the chaperonin CCT deficiency as a potential, yet unexplored, cause of neurodegenerative diseases.4
CCT participates in the folding of newly synthesized nascent polypeptides from ribosomes including actin, tubulin and several others. An increasing number of physiological CCT substrates have been identified; these include cyclin E, cdc20, pololike kinase 1 (PLK1), and Von Hippel-Lindau (VHL) tumor suppressor protein. Some data indicate that approximately 5-10% of newly synthesized proteins may flow through CCT. Many of these substrates are involved in cell cycle progression. Therefore, CCT plays an important role in cytoskeletal organization and cell division. Moreover, the protein folding activity of CCT might provide a mechanism to coordinate S6K- and RSK-regulated protein synthesis with protein folding. It was shown that RSK and S6K phosphorylate the CCTβ subunit at Ser-260. Furthermore, CCTβ plays an important role in regulating cell proliferation and Ser-260 phosphorylation contributes significantly to this process. Thus, the Ras-MAPK and PI3K-mTOR pathways utilize RSK and S6K to converge upon the phosphorylation and regulation of CCTβ function in mammalian cells.3
Recently, it was demonstrated that normal CCT function is ultimately required for the morphogenesis and survival of sensory neurons of the retina, and suggested the chaperonin CCT deficiency as a potential, yet unexplored, cause of neurodegenerative diseases.4
REFERENCES
1. Bregier, C. et al: Postepy. Biochem. 54:64-70, 2008
2. Willardson,, B. M. et al: Cell. Signal. 19:2417-27, 2007
3. Abe, Y. et al: J. Biol. Chem. 2284:14939-48, 2009
4. Posokhova, E. et al: Mol. Cell. Proteom. 10:M110.000570, 2011
2. Willardson,, B. M. et al: Cell. Signal. 19:2417-27, 2007
3. Abe, Y. et al: J. Biol. Chem. 2284:14939-48, 2009
4. Posokhova, E. et al: Mol. Cell. Proteom. 10:M110.000570, 2011
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10413 |
Antigen: | Raised against recombinant human TCP-1beta fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 54 kDa |
Specificity/Sensitivity: | Detects TCP-1beta proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой