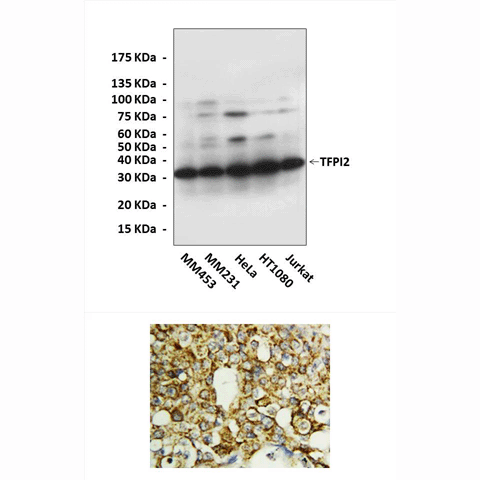
Anti-TFPI-2: Rabbit Tissue Factor Pathway Inhibitor-2 Antibody |
 |
BACKGROUND Tissue factor pathway inhibitor-2 (TFPI-2) is a 32 kDa matrix-associated Kunitz-type serine proteinase inhibitor consisting of a short amino-terminal region, three tandem Kunitz-type domains and a positively charged carboxyl-terminal tail. Human TFPI-2, previously designated as placental protein 5, inhibits a broad spectrum of serine proteinases almost exclusively through its first Kunitz-type domain, and is thought to play an important role in the regulation of extracellular matrix digestion and remodeling. In this context, reduced synthesis of TFPI-2 has been related to numerous pathophysiological processes such as inflammation, angiogenesis, atherosclerosis, retinal degeneration and tumor growth/metastasis.1
The expression of TFPI-2 in tumors is inversely related to an increasing degree of malignancy, which may suggest a role for TFPI-2 in the maintenance of tumor stability and inhibition of the growth of neoplasms. Clotting activation occurs frequently in cancer. Tissue factor (TF), the most potent initiator of coagulation, is expressed aberrantly in many types of malignancy and is involved not only in tumor-associated hypercoagulability but also in promoting tumor angiogenesis and metastasis via coagulation-dependent and coagulation-independent (signaling) mechanisms. TFPI-2 inhibits the tissue factor/factor VIIa (TF/VIIa) complex and a wide variety of serine proteinases including plasmin, plasma kallikrein, factor XIa, trypsin, and chymotrypsin. Studies have shown that TFPI exhibits antiangiogenic and antimetastatic effects in vitro and in vivo. In animal models of experimental metastasis, both circulating and tumor cell-associated TFPI are shown to significantly reduce tumor cell-induced coagulation activation and lung metastasis. Aberrant methylation of TFPI-2 promoter cytosine-phosphorothioate-guanine (CpG) islands in human cancers and cancer cell lines was widely documented to be responsible for diminished expression of mRNA encoding TFPI-2 and decreased or inhibited synthesis of TFPI-2 protein during cancer progression.2 Furthermore, an aberrantly spliced variant of TFPI-2 mRNA (designated asTFPI-2) was detected, which represents an untranslated form of TFPI-2. The levels of asTFPI-2 were very low or undetectable in normal cells but markedly upregulated in neoplastic tissue. TFPI-2 functions in the maintenance of the stability of the tumor environment and inhibits invasiveness and growth of neoplasms, as well as metastases formation. TFPI-2 has also been shown to induce apoptosis and inhibit angiogenesis, which may contribute significantly to tumor growth inhibition. Restoration of TFPI-2 expression in tumor tissue inhibits invasion, tumor growth, and metastasis, which creates a novel possibility of cancer patient treatment.3
The expression of TFPI-2 in tumors is inversely related to an increasing degree of malignancy, which may suggest a role for TFPI-2 in the maintenance of tumor stability and inhibition of the growth of neoplasms. Clotting activation occurs frequently in cancer. Tissue factor (TF), the most potent initiator of coagulation, is expressed aberrantly in many types of malignancy and is involved not only in tumor-associated hypercoagulability but also in promoting tumor angiogenesis and metastasis via coagulation-dependent and coagulation-independent (signaling) mechanisms. TFPI-2 inhibits the tissue factor/factor VIIa (TF/VIIa) complex and a wide variety of serine proteinases including plasmin, plasma kallikrein, factor XIa, trypsin, and chymotrypsin. Studies have shown that TFPI exhibits antiangiogenic and antimetastatic effects in vitro and in vivo. In animal models of experimental metastasis, both circulating and tumor cell-associated TFPI are shown to significantly reduce tumor cell-induced coagulation activation and lung metastasis. Aberrant methylation of TFPI-2 promoter cytosine-phosphorothioate-guanine (CpG) islands in human cancers and cancer cell lines was widely documented to be responsible for diminished expression of mRNA encoding TFPI-2 and decreased or inhibited synthesis of TFPI-2 protein during cancer progression.2 Furthermore, an aberrantly spliced variant of TFPI-2 mRNA (designated asTFPI-2) was detected, which represents an untranslated form of TFPI-2. The levels of asTFPI-2 were very low or undetectable in normal cells but markedly upregulated in neoplastic tissue. TFPI-2 functions in the maintenance of the stability of the tumor environment and inhibits invasiveness and growth of neoplasms, as well as metastases formation. TFPI-2 has also been shown to induce apoptosis and inhibit angiogenesis, which may contribute significantly to tumor growth inhibition. Restoration of TFPI-2 expression in tumor tissue inhibits invasion, tumor growth, and metastasis, which creates a novel possibility of cancer patient treatment.3
REFERENCES
1. Chand, H.S. et al: Thromb Haemost. 94:1122-30, 2005
2. Takada, H. et al: Cancer Genet. Cytogenet.197:16-24, 2010
3. Sierko, E. et al: Semin. Thromb. Hemost. 33:653-9, 2007
2. Takada, H. et al: Cancer Genet. Cytogenet.197:16-24, 2010
3. Sierko, E. et al: Semin. Thromb. Hemost. 33:653-9, 2007
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1248 |
Antigen: | Short peptide from human TFPI-2 sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 32 kDa |
Specificity/Sensitivity: | Detects endogenous levels of TFPI-2 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой