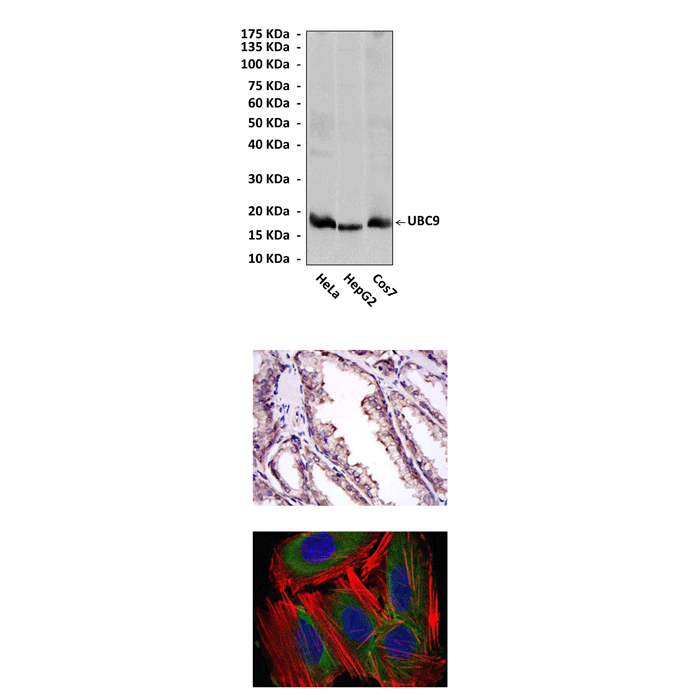
Anti-UBC9/UBE2I: Mouse UBC9/UBE2I Antibody |
 |
BACKGROUND SUMO conjugation or sumoylation is similar to ubiquitination in structure, conjugation process, and attachment to target proteins. However, the biological consequences of these two pathways can be quite distinct. Unlike ubiquitination that normally targets proteins for degradation through proteasome pathways, sumoylation has been implicated in the regulation of protein stability, protein-protein interactions, transcriptional activity, and sUBCellular localization.1
UBC9 is an E2-conjugating enzyme essential for sumoylation, and it transfers the activated SUMO to protein substrates. In particular, UBC9 has been shown to play a key role in nuclear trafficking, transcriptional regulation, and protein stability through regulation of the sumoylation machinery. In addition, studies indicated that UBC9 is a multifunctional protein that can exert its functions independent of sumoylation. Many important proteins, including tumor suppressors and oncoproteins, as well as the cell cycle and proliferation-related proteins, are targets for sumoylation or interact with UBC9; their expression or their activity is regulated by UBC9. Thus, alterations of UBC9 could ultimately have an impact on cell growth and cancer development. UBC-9 activity is required for several developmental processes including embryogenesis, and the DNA damage response (both DNA repair and DNA damage checkpoint function); UBC-9 interacts with LIN-1, an ETS-domain-containing transcription factor that negatively regulates vulval development. Additionally, several studies indicate that UBC9 plays a role in tumorigenesis and drug responsiveness. Furthermore, autosumoylation of UBC9 at Lys14 regulates target discrimination.2 While not altering its activity toward HDAC4, E2-25K, PML, or TDG, sumoylation of UBC9 impairs its activity on RanGAP1 and strongly activates sumoylation of the transcriptional regulator Sp100. Enhancement depends on a SUMO-interacting motif (SIM) in Sp100 that creates an additional interface with the SUMO conjugated to the E2, a mechanism distinct from UBC9∼SUMO thioester recruitment.3
UBC9 is a single-copy gene and is ubiquitously expressed in all human organs and tissues. However, levels of UBC9 vary in different organs or tissues. In tumors UBC9 is frequently up-regulated. For example, UBC9 is up-regulated in lung adenocarcinoma, as detected by microarray analysis. It was shown that UBC9 expression is subjected to microRNA regulation at the posttranscriptional level, where miR-30e negatively regulates UBC9 expression by translation repression, which may be responsible for the frequent UBC9 overexpression in tumors.4
UBC9 is an E2-conjugating enzyme essential for sumoylation, and it transfers the activated SUMO to protein substrates. In particular, UBC9 has been shown to play a key role in nuclear trafficking, transcriptional regulation, and protein stability through regulation of the sumoylation machinery. In addition, studies indicated that UBC9 is a multifunctional protein that can exert its functions independent of sumoylation. Many important proteins, including tumor suppressors and oncoproteins, as well as the cell cycle and proliferation-related proteins, are targets for sumoylation or interact with UBC9; their expression or their activity is regulated by UBC9. Thus, alterations of UBC9 could ultimately have an impact on cell growth and cancer development. UBC-9 activity is required for several developmental processes including embryogenesis, and the DNA damage response (both DNA repair and DNA damage checkpoint function); UBC-9 interacts with LIN-1, an ETS-domain-containing transcription factor that negatively regulates vulval development. Additionally, several studies indicate that UBC9 plays a role in tumorigenesis and drug responsiveness. Furthermore, autosumoylation of UBC9 at Lys14 regulates target discrimination.2 While not altering its activity toward HDAC4, E2-25K, PML, or TDG, sumoylation of UBC9 impairs its activity on RanGAP1 and strongly activates sumoylation of the transcriptional regulator Sp100. Enhancement depends on a SUMO-interacting motif (SIM) in Sp100 that creates an additional interface with the SUMO conjugated to the E2, a mechanism distinct from UBC9∼SUMO thioester recruitment.3
UBC9 is a single-copy gene and is ubiquitously expressed in all human organs and tissues. However, levels of UBC9 vary in different organs or tissues. In tumors UBC9 is frequently up-regulated. For example, UBC9 is up-regulated in lung adenocarcinoma, as detected by microarray analysis. It was shown that UBC9 expression is subjected to microRNA regulation at the posttranscriptional level, where miR-30e negatively regulates UBC9 expression by translation repression, which may be responsible for the frequent UBC9 overexpression in tumors.4
REFERENCES
1. Verger, A. et al: EMBO Repot. 4:137-42, 2003
2. Knipscheer, P. et al: Mol. Cell 31:371-82, 2008
3. Subramaniam, S. et al: J. Biol. Chem. 285:20428-32,2010
4. Wu, F. et al: Clin. Cancer Res. 15:1550-7, 2009
2. Knipscheer, P. et al: Mol. Cell 31:371-82, 2008
3. Subramaniam, S. et al: J. Biol. Chem. 285:20428-32,2010
4. Wu, F. et al: Clin. Cancer Res. 15:1550-7, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10431 |
Antigen: | Raised against recombinant human UBC9 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP 1:50 IHC 1:50 - 1:200 ICC n/d FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 18 kDa |
Specificity/Sensitivity: | Detects endogenous UBC9 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой