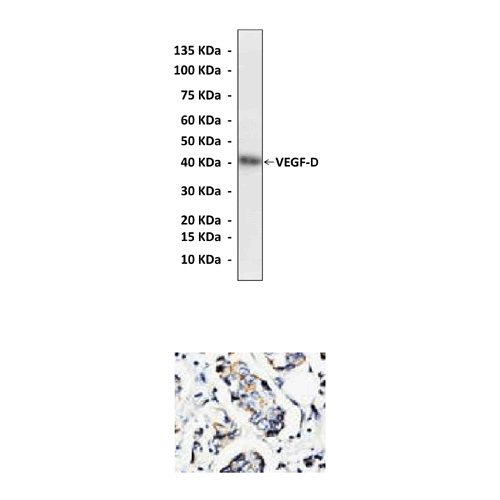
Anti-VEGF-D: Poyclonal Vascular Endothelial Growth Factor-D Antibody |
 |
BACKGROUND Vascular endothelial growth factor (VEGF) is an important signaling protein involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature). VEGF has several splicing isoforms (in humans: VEGF121, VEGF121b, VEGF145, VEGF165, VEGF165b, VEGF189, VEGF206; the rodent orthologs of these proteins contain one fewer amino acid). They belong to a growth factor family containing other closely-related proteins (PIGF, VEGF-B, VEGF-C, VEGF-D, and PIGF).1 VEGF is sometimes referred to as VEGF-A to differentiate it from these related growth factors. A number of VEGF-related proteins have also been discovered encoded by viruses (VEGF-E) and in the venom of some snakes (VEGF-F).2
All members of the VEGF family stimulate cellular responses by binding to tyrosine kinase receptors (the VEGFRs) on the cell surface. The ligand binding leads to dimerization of receptors and activation of their kinase activity. The activated receptor tyrosine kinases interact with and phosphorylate downstream signaling components including PLC-gamma, p85, and SHC etc, which mediate VEGF-signaling in the cells.3,4
VEGF-C and VEGF-D (also known as c-fos-induced growth factor) are the only known ligands for VEGFR-3. VEGF-D is angiogenic, mitogenic for endothelial cells in vitro, is expressed at many sites in the developing embryo, and is localized in human tumors. VEGF-D also induced lymphangiogenesis and metastatic spread via the lymphatics in a mouse tumor model. VEGF-D is initially synthesized as a precursor protein containing N- and C-terminal propeptides in addition to the VEGF homology domain (VHD), the region of the protein that shares homology with all VEGF family members and contains receptor-binding epitopes. The N- and C-terminal propeptides are proteolytically cleaved from the VHD during biosynthesis to generate a mature, secreted form consisting of dimers of the VHD. The mature form of human VEGF-D binds both VEGFR-2 and VEGFR-3 with much higher affinity than does unprocessed VEGF-D. Therefore proteolytic processing is important for activating human VEGF-D. As human VEGF-D activates VEGFR-2 and VEGFR-3, it has been proposed that VEGF-D can stimulate the growth of blood vessels and lymphatic vessels into regions of developing embryos and tumors.5
All members of the VEGF family stimulate cellular responses by binding to tyrosine kinase receptors (the VEGFRs) on the cell surface. The ligand binding leads to dimerization of receptors and activation of their kinase activity. The activated receptor tyrosine kinases interact with and phosphorylate downstream signaling components including PLC-gamma, p85, and SHC etc, which mediate VEGF-signaling in the cells.3,4
VEGF-C and VEGF-D (also known as c-fos-induced growth factor) are the only known ligands for VEGFR-3. VEGF-D is angiogenic, mitogenic for endothelial cells in vitro, is expressed at many sites in the developing embryo, and is localized in human tumors. VEGF-D also induced lymphangiogenesis and metastatic spread via the lymphatics in a mouse tumor model. VEGF-D is initially synthesized as a precursor protein containing N- and C-terminal propeptides in addition to the VEGF homology domain (VHD), the region of the protein that shares homology with all VEGF family members and contains receptor-binding epitopes. The N- and C-terminal propeptides are proteolytically cleaved from the VHD during biosynthesis to generate a mature, secreted form consisting of dimers of the VHD. The mature form of human VEGF-D binds both VEGFR-2 and VEGFR-3 with much higher affinity than does unprocessed VEGF-D. Therefore proteolytic processing is important for activating human VEGF-D. As human VEGF-D activates VEGFR-2 and VEGFR-3, it has been proposed that VEGF-D can stimulate the growth of blood vessels and lymphatic vessels into regions of developing embryos and tumors.5
REFERENCES
1. Neufeld, G. et al. FASEB J. 13:9, 1999.
2. Yamazaki, Y. & Morita, T. : Mol. Divers. 10:215, 2006.
3. Shapiro, S. D.: J Clin Invest.106: 1309, 2000.
4. Meyer, R. D. et al. : J. Biol. Chem. 277: 27081, 2002.
5. Achen, M.G. et al: proc. Natl. Acad. Sci. USA 95:548-53, 1998
2. Yamazaki, Y. & Morita, T. : Mol. Divers. 10:215, 2006.
3. Shapiro, S. D.: J Clin Invest.106: 1309, 2000.
4. Meyer, R. D. et al. : J. Biol. Chem. 277: 27081, 2002.
5. Achen, M.G. et al: proc. Natl. Acad. Sci. USA 95:548-53, 1998
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1461 |
Antigen: | C-terminal sequence of human VEGF-D |
Isotype: | Affinity-purified rabbit polyclonal IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat, Rabbit |
Applications & Suggested starting dilutions: * | WB 1:500 to 1:1000 IP n/d IHC (Paraffin) 1:50 to 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 40 kDa |
Specificity/Sensitivity: | Reacts specifically with VEGF-D of human, rabbit, mouse & rat origin in immunostaining and western blotting, no cross-reactivity with other members of the family. |
Storage: | Store at 4° C for frequent use; at -20° C for at least one year. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой