IFN-γ ELISA Kit, Human |
 |
BACKGROUND IFN-gamma is the sole type II IFN. It is structurally unrelated to type I IFNs, binds to a different receptor, and is encoded by a separate chromosomal locus. Although IFN-gamma has some antiviral activity, it is much less active in this regard than type I IFNs. IFN-gamma is involved in the regulation of nearly all phases of the immune and inflammatory responses, including the activation and differentiation of T cells, B cells, NK cells, macrophages, and others. It is therefore best regarded as a distint immunoregulatory cytokine.1 Indeed, IFN-gamma serves critical functions in innate immunity and in specific cell-mediated immunity (in addition, IFN activates neutrophilis and stimulates the cytolitic activity of NK cells). Many IFN-gamma induced effects result in heigtened immune surveillance. IFN-gamma can promote macrophage activation, mediate antiviral e antibacterial immunity, enhance antigen presentation, orchestrate activation of the innate immune system, coordinate lymphocyte-endothelium interaction, regulate Th1/Th2 balance, and control cellular proliferation and apoptosis. IFN-gamma secretion is a hallmark of Th1 lymphocytes. It is also secreted by nearly all CD8 T cells, by some Th0 cells, professional antigen-presenting cells (APCs), and by NK cells. Each of these cell types secretes IFN-gamma only when activated, usually as part of immune response and especially in response to IL-2 and IL-12. IFN-gamma production is inhibited by IL-4, IL-10, TGF-beta, glucocorticoids, cyclosporin A and FK506.2
Functional IFN-gamma receptor (IFNGR) is comprised of two ligand-binding IFNGR1 chains associated with two signal-transducing IFNGR2 chains and associated signaling machinery. Ligand binding causes a conformational change in the IFN-gammaR (IFNGR1, IFNGR2), such that the inactive Jak2 kinase undergoes autophosphorylation and activation, which in turn allows Jak1 transphosphorylation by Jak2. The activated Jak1 phosphorylates functionally critical Tyr440 of each IFNGR1 chain to form two adjacent docking sites for the Src homology (SH)2 domains of latent Stat1. The receptor-recruited Stat1 pair is phosphorylated near the C terminus at Tyr701. Phosphorylation induces dissociation of a Stat1 homodimer from the receptor. To a lesser extent, IFN-gamma signaling also produces Stat1:Stat1:IFN regulatory factor (IRF)-9 and Stat1:Stat2:IRF-9 [IFN-stimulated gene factor 3 (ISGF3)] complexes. Stat1 homodimers travel to the nucleus and bind to promoter IFN-gamma-activation site (GAS) elements to initiate/suppress transcription of IFN-gamma-regulated genes.3 Many of IFN-gamma-regulated genes are in fact transcription factors (e.g., IRF-1), which are activated by IFN-gamma and are able to drive regulation of the next wave of transcription (e.g., induction of IFN-beta).
Functional IFN-gamma receptor (IFNGR) is comprised of two ligand-binding IFNGR1 chains associated with two signal-transducing IFNGR2 chains and associated signaling machinery. Ligand binding causes a conformational change in the IFN-gammaR (IFNGR1, IFNGR2), such that the inactive Jak2 kinase undergoes autophosphorylation and activation, which in turn allows Jak1 transphosphorylation by Jak2. The activated Jak1 phosphorylates functionally critical Tyr440 of each IFNGR1 chain to form two adjacent docking sites for the Src homology (SH)2 domains of latent Stat1. The receptor-recruited Stat1 pair is phosphorylated near the C terminus at Tyr701. Phosphorylation induces dissociation of a Stat1 homodimer from the receptor. To a lesser extent, IFN-gamma signaling also produces Stat1:Stat1:IFN regulatory factor (IRF)-9 and Stat1:Stat2:IRF-9 [IFN-stimulated gene factor 3 (ISGF3)] complexes. Stat1 homodimers travel to the nucleus and bind to promoter IFN-gamma-activation site (GAS) elements to initiate/suppress transcription of IFN-gamma-regulated genes.3 Many of IFN-gamma-regulated genes are in fact transcription factors (e.g., IRF-1), which are activated by IFN-gamma and are able to drive regulation of the next wave of transcription (e.g., induction of IFN-beta).
REFERENCES
1. Schroder, K. et al: J. Leuk. Biol. 75:163-189, 2004
2. Boehm, U. et al: Annu Rev Immunol. 15:749-95, 1997
3. Ramana, C.V. et al: Trends Immunol. 23:96-101, 2002
2. Boehm, U. et al: Annu Rev Immunol. 15:749-95, 1997
3. Ramana, C.V. et al: Trends Immunol. 23:96-101, 2002
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0373 |
Target Protein Species: | Human |
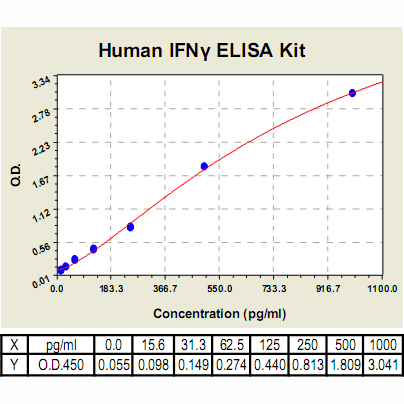
Range: | 15.6 pg/ml – 1000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой