LOX-1 ELISA Kit, Mouse |
 |
BACKGROUND Lectin-like oxidized LDL receptor-1 (LOX-1) is a disulfide-linked homodimeric type II transmembrane protein with a short 34-residue cytoplasmic tail, a single transmembrane domain, and an extracellular region consisting of an 80-residue domain predicted to be a coil followed by a 130-residue C-terminal C-type lectin-like domain (CTLD) responsible for ox-LDL recognition. It belongs to the class E scavenger receptor family, a structurally diverse group of cell surface receptors of the innate immune system that recognize and internalize oxidized LDL (ox-LDL) in endothelial cells of large arteries. More recent studies have indicated that LOX-1 is expressed in other cells types, including macrophages, vascular smooth-muscle cells, and platelets. Its expression is not constitutive, but rather, markedly induced by proinflammatory, oxidative, and mechanical stimuli, which leads to the activation of endothelial cells, transformation of smooth-muscle cells, and accumulation of lipids in macrophages, resulting in cellular injury and the development of atherosclerosis.1
It has been demonstrated that oxLDL-mediated activation triggers an endothelial LOX-1-linked signaling pathway leading to pro-inflammatory responses. LOX-1 activation stimulates ROS production and activates NF-κB. NF-κB activation and translocation into the nucleus results in increased transcription of genes encoding pro-inflammatory and adhesion molecules, e.g. tumor necrosis factor-α, ICAM-1, and VCAM-1. A different signaling pathway in vascular smooth muscle cells (VSMCs) may also be regulated by LOX-1 activation. VSMC differentiation, migration, and proliferation regulate the formation of the fibrous cap and ECM of atherosclerotic plaques. Moreover, LOX-1 activation by oxLDL stimulates the p38 MAPK intracellular signaling pathway, increasing endothelial MMP gene expression, secretion, and activity in vitro. The CD40-CD40L receptor-ligand system regulates this pathway in VSMCs by inducing pro-inflammatory gene expression including cytokines, proteinases, and adhesion molecules. Recent findings suggest that LOX-1 activation elevates the CD40-CD40L pathophysiological response in VSMCs. The shedding of endothelial membrane proteins as soluble fragments into extracellular fluids are potential vascular disease biomarkers.2 Soluble circulatory polypeptides derived from adhesion proteins, e.g. VCAM-1 and ICAM-1, are generated by post-translational processing. LOX-1 also undergoes post-translational proteolytic cleavage within the “neck” region of the extracellular domain releasing a 187-residue polypeptide containing the C-type lectin-like domain known as sLOX-1. Mammalian sLOX-1 is a potentially exciting biomarker in vascular disease prediction. The generation of a wider range of antibody and ligand-based assays to monitor membrane-bound or sLOX-1 in the vascular tissues or in body fluids could allow better diagnosis of different vascular and pro-inflammatory diseases.3
It has been demonstrated that oxLDL-mediated activation triggers an endothelial LOX-1-linked signaling pathway leading to pro-inflammatory responses. LOX-1 activation stimulates ROS production and activates NF-κB. NF-κB activation and translocation into the nucleus results in increased transcription of genes encoding pro-inflammatory and adhesion molecules, e.g. tumor necrosis factor-α, ICAM-1, and VCAM-1. A different signaling pathway in vascular smooth muscle cells (VSMCs) may also be regulated by LOX-1 activation. VSMC differentiation, migration, and proliferation regulate the formation of the fibrous cap and ECM of atherosclerotic plaques. Moreover, LOX-1 activation by oxLDL stimulates the p38 MAPK intracellular signaling pathway, increasing endothelial MMP gene expression, secretion, and activity in vitro. The CD40-CD40L receptor-ligand system regulates this pathway in VSMCs by inducing pro-inflammatory gene expression including cytokines, proteinases, and adhesion molecules. Recent findings suggest that LOX-1 activation elevates the CD40-CD40L pathophysiological response in VSMCs. The shedding of endothelial membrane proteins as soluble fragments into extracellular fluids are potential vascular disease biomarkers.2 Soluble circulatory polypeptides derived from adhesion proteins, e.g. VCAM-1 and ICAM-1, are generated by post-translational processing. LOX-1 also undergoes post-translational proteolytic cleavage within the “neck” region of the extracellular domain releasing a 187-residue polypeptide containing the C-type lectin-like domain known as sLOX-1. Mammalian sLOX-1 is a potentially exciting biomarker in vascular disease prediction. The generation of a wider range of antibody and ligand-based assays to monitor membrane-bound or sLOX-1 in the vascular tissues or in body fluids could allow better diagnosis of different vascular and pro-inflammatory diseases.3
REFERENCES
1. Reiss, A.B. et al: Curr. Med. Chem. 16:2641-52, 2009
2. Dunn, S. et al: Biochem. J. 409:349-55, 2008
3. Mehta, J.L. & Li, D.: J. Am. Coll. Cardiol. 39:1429-35, 2002
2. Dunn, S. et al: Biochem. J. 409:349-55, 2008
3. Mehta, J.L. & Li, D.: J. Am. Coll. Cardiol. 39:1429-35, 2002
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0825 |
Target Protein Species: | Mouse |
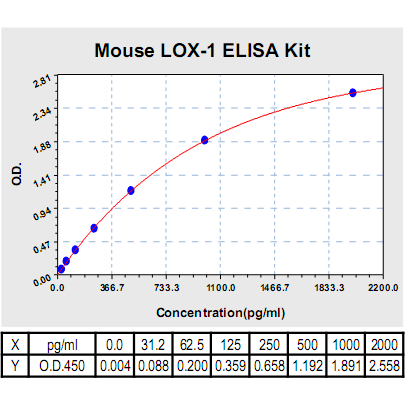
Range: | 31.2pg/ml-2000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokine. |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой