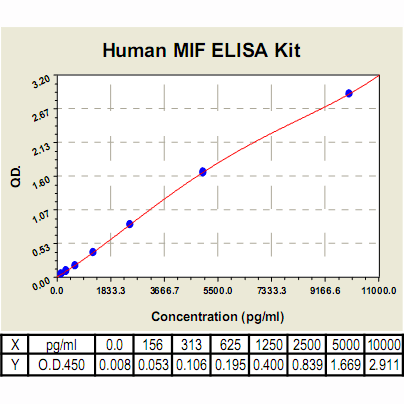
MIF ELISA Kit, Human |
 |
BACKGROUND Macrophage migration inhibitory factor (MIF) functions as a pleiotropic protein, participating in inflammatory and immune responses. MIF was originally discovered as a lymphokine involved in delayed hypersensitivity and various macrophage functions, including phagocytosis, spreading, and tumoricidal activity. Furthermore, it has been demonstrated that MIF was a proinflammatory cytokine and pituitary-derived hormone potentiating endotoxemia. This protein is ubiquitously expressed in various organs, such as the brain and kidney. It is produced constitutively by diverse cell types, and it circulates normally in the blood at low but immunoregulatory concentrations. MIF is not only secreted by immune cells, but also by parenchymal and tumour cells upon inflammatory and stress stimulation.1 Among cytokines, MIF is unique in terms of its abundant expression and storage within the cytoplasm, and, further, for its counteraction against glucocorticoids. Diverse pro-inflammatory or invasive stimuli lead to a rapid upregulation in the release of MIF from pre-formed stores in monocytes/macrophages and other cell types. MIF has unexpectedly been found to convert D-dopachrome, an enantiomer of naturally occurring L-dopachrome, to 5,6-dihydroxyindole. It was demonstrated that anti-MIF antibodies effectively suppress tumor growth and tumor-associated angiogenesis, suggesting that MIF is involved not only in inflammatory and immune responses but also in tumor cell growth. Thus, MIF cannot be clearly categorized as either a cytokine, hormone, or enzyme.2
MIF is an upstream regulator of the host immunity that promotes cellular inflammatory responses such as mitogen-activated protein kinase (MAPK) signalling, tumour necrosis factor-α (TNF-α) secretion or cyclooxygenase-2 (COX-2) activity. Owing to its inflammatory activities, MIF is a pivotal mediator of acute and chronic inflammatory diseases including septic shock, rheumatoid arthritis, and atherosclerosis. Sharing an architectural 3D similarity with the atherogenic and angiogenic chemokine interleukin-8 (IL-8)/CXCL8, MIF was found to function as a non-cognate ligand of CXCR2 and as chemokine-like function (CLF) chemokine. Inflammatory leukocyte recruitment is dependent on MIF-CXCR2 and MIF-CXCR4 interactions. Additionally, MIF exerts at least some of its biological actions through binding to its extracellular cognate receptor complex consisting of CD74 and CD44. Studies have revealed a requirement for extracellular MIF in the steady-state activation of Rho GTPase family members, leading to cell growth and migratory phenotypes. As observed for CLF chemokines, MIF action is not limited to the extracellular space, but also occurs intracellularly. MIF is found in the cytosol of various cell types, where it contributes to cell survival, cell cycle and homeostasis control. Intracellular MIF activities are linked to c-Jun activation domain binding protein-1 (JAB1), the tumour suppressor protein p53, and the thiolprotein oxidoreductase (TPOR) activity of MIF.3
MIF is an upstream regulator of the host immunity that promotes cellular inflammatory responses such as mitogen-activated protein kinase (MAPK) signalling, tumour necrosis factor-α (TNF-α) secretion or cyclooxygenase-2 (COX-2) activity. Owing to its inflammatory activities, MIF is a pivotal mediator of acute and chronic inflammatory diseases including septic shock, rheumatoid arthritis, and atherosclerosis. Sharing an architectural 3D similarity with the atherogenic and angiogenic chemokine interleukin-8 (IL-8)/CXCL8, MIF was found to function as a non-cognate ligand of CXCR2 and as chemokine-like function (CLF) chemokine. Inflammatory leukocyte recruitment is dependent on MIF-CXCR2 and MIF-CXCR4 interactions. Additionally, MIF exerts at least some of its biological actions through binding to its extracellular cognate receptor complex consisting of CD74 and CD44. Studies have revealed a requirement for extracellular MIF in the steady-state activation of Rho GTPase family members, leading to cell growth and migratory phenotypes. As observed for CLF chemokines, MIF action is not limited to the extracellular space, but also occurs intracellularly. MIF is found in the cytosol of various cell types, where it contributes to cell survival, cell cycle and homeostasis control. Intracellular MIF activities are linked to c-Jun activation domain binding protein-1 (JAB1), the tumour suppressor protein p53, and the thiolprotein oxidoreductase (TPOR) activity of MIF.3
REFERENCES
1. Javeed, A. et al: Inflamm. Res. 57:45-50, 2008
2. Nishihira, J.: J. Interfer. Cytok. Res. 20: 751-62, 2000
3. Nishihira, J. et al: Ann. N Y Acad. Sci. 995:171-82, 2003
2. Nishihira, J.: J. Interfer. Cytok. Res. 20: 751-62, 2000
3. Nishihira, J. et al: Ann. N Y Acad. Sci. 995:171-82, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0813 |
Target Protein Species: | Human |
Range: | 156 pg/ml – 10000pg/ml |
Specificity: | No detectable cross-reactivity with other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой