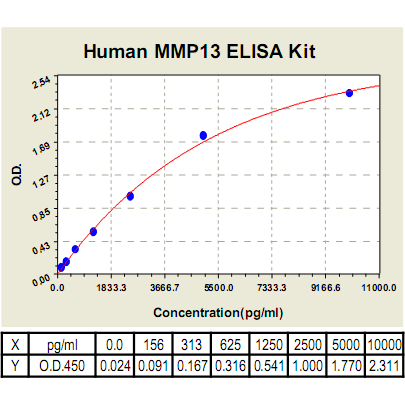
MMP-13 ELISA Kit, Human |
 |
BACKGROUND MMPs (matrix metalloproteinases) are zinc-dependent endopeptidases that are mainly involved in turnover and proteolytic degradation of the extracellular matrix and are believed to play a role in tissue remodeling in a number of normal and pathological conditions. MMPs may play a key role in physiologic and pathologic remodeling of tissues, including embryogenesis and tissue morphogenesis, wound repair, inflammatory diseases, cancerogenesis, etc. These multiple actions of MMPs are due to their ability to degrade extracellular matrices, basement membranes, and other proteins. So far, more than 25 members of the MMP family have been identified, and they can be divided into four groups on the basis of their domain structure, substrate specificity and cellular localization. These four groups are collagenases (MMP-1, -8 and -13), stromelysins (MMP-3, -7, -10, -11 and -12), gelatinases (MMP-2 and -9) and membrane-type MMPs.1
MMP-13 is a glycosylated protein of 60 kDa. It was reported that MMP-13 is tightly bound to tissues and utilizes heparan sulfate proteoglycans as extracellular docking molecules. MMP-13 is produced by many different cell types as an inactive proform that requires proteolytic activation to gain activity. Activation can involve MMP-2 and MMP-14. Plasmin is also an activating factor of the MMP-13 proform, but Plasmin can further deactivate active MMP-13. Trypsin-2 was found to directly activate MMP-13. MMP-13 has a wide substrate specificity, degrading collagen type 1, collagen type 3, collagen type 4. MMP-13 preferentially hydrolyzes soluble collagen type 2. Fibrillar collagen type 1 is cleaved with efficiencies comparable to that of MMP-1 and MMP-8. Other substrates are aggrecan, gelatin, Galectin-3, the proform of MMP-9. It was also reported that MMP-13 can proteolytically process fibrinogen and also inactivates Factor XII (Hageman factor). MMP-13 was found to degrade fibrillin, the principal structural component of the 10-12 nm diameter elastic microfibrils of the extracellular matrix. MMP-13 is inhibited by the tissue inhibitors of metalloproteinases, TIMP-1, TIMP-2, and TIMP-3.2
MMP-13 has been shown to be produced by a variety of normal and tumor cells including Kupffer cells, fibroblasts, synoviocytes, chondrocytes, smooth muscle cells, osteoblasts, neurons, tooth pulp cells, endothelial cells, granulosa cells, Theca cells, and human transformed epidermal keratinocytes etc. It has been shown that MMP-13 binds to, and is internalized by, a specific receptor, which has been identified as ENDO180 (CD280). MMP-13, together with other metalloproteinases, degrades major components of the extracellular matrix and thus can be assumed to play a role in skeletal development, tissue remodeling, and wound healing. MMP-13, as one of the collagenases, is responsible for collagen remodeling associated with angiogenesis.3
MMP-13 is a glycosylated protein of 60 kDa. It was reported that MMP-13 is tightly bound to tissues and utilizes heparan sulfate proteoglycans as extracellular docking molecules. MMP-13 is produced by many different cell types as an inactive proform that requires proteolytic activation to gain activity. Activation can involve MMP-2 and MMP-14. Plasmin is also an activating factor of the MMP-13 proform, but Plasmin can further deactivate active MMP-13. Trypsin-2 was found to directly activate MMP-13. MMP-13 has a wide substrate specificity, degrading collagen type 1, collagen type 3, collagen type 4. MMP-13 preferentially hydrolyzes soluble collagen type 2. Fibrillar collagen type 1 is cleaved with efficiencies comparable to that of MMP-1 and MMP-8. Other substrates are aggrecan, gelatin, Galectin-3, the proform of MMP-9. It was also reported that MMP-13 can proteolytically process fibrinogen and also inactivates Factor XII (Hageman factor). MMP-13 was found to degrade fibrillin, the principal structural component of the 10-12 nm diameter elastic microfibrils of the extracellular matrix. MMP-13 is inhibited by the tissue inhibitors of metalloproteinases, TIMP-1, TIMP-2, and TIMP-3.2
MMP-13 has been shown to be produced by a variety of normal and tumor cells including Kupffer cells, fibroblasts, synoviocytes, chondrocytes, smooth muscle cells, osteoblasts, neurons, tooth pulp cells, endothelial cells, granulosa cells, Theca cells, and human transformed epidermal keratinocytes etc. It has been shown that MMP-13 binds to, and is internalized by, a specific receptor, which has been identified as ENDO180 (CD280). MMP-13, together with other metalloproteinases, degrades major components of the extracellular matrix and thus can be assumed to play a role in skeletal development, tissue remodeling, and wound healing. MMP-13, as one of the collagenases, is responsible for collagen remodeling associated with angiogenesis.3
REFERENCES
1. Mohamed, A.J. et al: Immunol. Rev. 228:58-73, 2009
2. Knäuper, V. et al: J. Biol. Chem. 271:17124-31, 1996
3. Hattori, N. et al: Am. J. Path. 175:533-46, 2009
2. Knäuper, V. et al: J. Biol. Chem. 271:17124-31, 1996
3. Hattori, N. et al: Am. J. Path. 175:533-46, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0468 |
Target Protein Species: | Human |
Range: | 78pg/ml→5000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokine. |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой