OPG ELISA Kit, Mouse |
 |
BACKGROUND Osteoprotegerin (OPG), a member of the tumor necrosis factor receptor superfamily, is a soluble decoy receptor for the osteoclast differentiation factor receptor-activator of nuclear factor B ligand (RANKL) that inhibits interaction between RANKL and its membrane-bound receptor RANK. OPG protein comprises 401 amino acid residues of which 21 form a signal peptide that is cleaved generating a mature form of 380 amino acid residues. At the N-terminus, there are four domains (D1-4), which have Cys-rich TNFR homologue mortifs and are necessary and sufficient for binding to RANKL, and for inhibiting osteoclastic differentiation and acitivity. At the C-terminus, there are tandem death-domain homologue regions (D5 and D6) followed by a heparin-binding site (D7) and , at position 400, there is a Cys residue required for homodimerization of OPG. OPG is produced as a monomer (55-62 kDa), undergoes homodimerization, and is secreted as a disulfide-linked homodimeric glycoprotein with four or five glycosylation sites, generating a mature form of OPG of 110-120 kDa. The dimeric form of OPG exhibits a much higher affinity to RANKL.1
The RANKL/OPG/RANK axis has been shown to regulate bone remodeling. RANKL is essential for osteoclast formation, function and survival and each of these activities are prevented by OPG. OPG has traditionally been linked to a number of bone-related diseases. However, there is additional evidence that OPG can promote cell survival by inhibiting TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. As a result, a number of in vitro, in vivo and clinical studies have been performed assessing the role of OPG in tumourigenesis. Similar studies have been performed regarding vascular pathologies, resulting from observations of expression and regulation of OPG in the vasculature.2 It has been demonstrated that RANKL/OPG/RANK system is linked to the development of atherosclerosis and plaque destabilization. RANKL exhibits several properties with relevance to atherogenesis, such as promotion of inflammatory responses in T cells and dendritic cells, induction of chemotactic properties in monocytes, induction of matrix metalloproteinase (MMP) activity in vascular smooth muscle cells (SMC), and RANKL has also been found to have prothrombotic properties. In observational studies, elevated circulating OPG levels have been associated with prevalence and severity of coronary artery disease, cerebrovascular disease, and peripheral vascular disease. Circulating OPG levels are increased in patients with acute coronary syndrome, and enhanced expression has been found within symptomatic carotid plaques. Elevated OPG levels have also been associated with the degree of coronary calcification in the general population as a marker of coronary atherosclerosis. OPG has been reported to predict survival in patients with heart failure after acute myocardial infarction, to predict heart failure hospitalization and mortality in patients with acute coronary syndrome, and to be associated with long-term mortality in patients with ischemic stroke. There are also a few studies that show a relationship between OPG and cardiovascular disease (CVD) and related mortality in the general population.3 Additionally, the RANKL/OPG/RANK axis was found to be involved in central thermoregulation.4
The RANKL/OPG/RANK axis has been shown to regulate bone remodeling. RANKL is essential for osteoclast formation, function and survival and each of these activities are prevented by OPG. OPG has traditionally been linked to a number of bone-related diseases. However, there is additional evidence that OPG can promote cell survival by inhibiting TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. As a result, a number of in vitro, in vivo and clinical studies have been performed assessing the role of OPG in tumourigenesis. Similar studies have been performed regarding vascular pathologies, resulting from observations of expression and regulation of OPG in the vasculature.2 It has been demonstrated that RANKL/OPG/RANK system is linked to the development of atherosclerosis and plaque destabilization. RANKL exhibits several properties with relevance to atherogenesis, such as promotion of inflammatory responses in T cells and dendritic cells, induction of chemotactic properties in monocytes, induction of matrix metalloproteinase (MMP) activity in vascular smooth muscle cells (SMC), and RANKL has also been found to have prothrombotic properties. In observational studies, elevated circulating OPG levels have been associated with prevalence and severity of coronary artery disease, cerebrovascular disease, and peripheral vascular disease. Circulating OPG levels are increased in patients with acute coronary syndrome, and enhanced expression has been found within symptomatic carotid plaques. Elevated OPG levels have also been associated with the degree of coronary calcification in the general population as a marker of coronary atherosclerosis. OPG has been reported to predict survival in patients with heart failure after acute myocardial infarction, to predict heart failure hospitalization and mortality in patients with acute coronary syndrome, and to be associated with long-term mortality in patients with ischemic stroke. There are also a few studies that show a relationship between OPG and cardiovascular disease (CVD) and related mortality in the general population.3 Additionally, the RANKL/OPG/RANK axis was found to be involved in central thermoregulation.4
REFERENCES
1. D'Amelio, P. et al: J Endocrinol Invest. 32(4 Suppl):6-9, 2009
2. Kiechl, S. et al: Expert Rev Cardiovasc Ther. 4:801-11, 2006
3. Caidahl, K. et al: Arterioscler Thromb Vasc Biol. 30:1684-6, 2010
4. Hanada, R. et al: Nature 462: 505–9,2009
2. Kiechl, S. et al: Expert Rev Cardiovasc Ther. 4:801-11, 2006
3. Caidahl, K. et al: Arterioscler Thromb Vasc Biol. 30:1684-6, 2010
4. Hanada, R. et al: Nature 462: 505–9,2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0481 |
Target Protein Species: | Mouse |
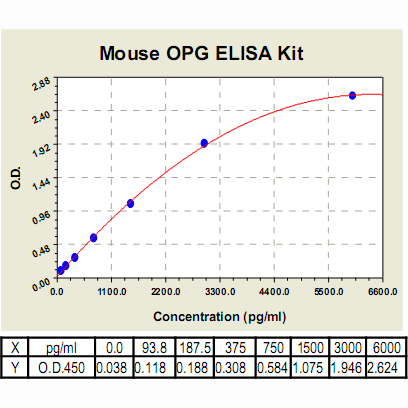
Range: | 93.8 pg/ml – 6000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой