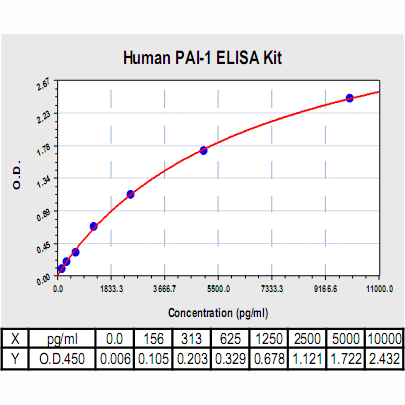
PAI-1 ELISA Kit, Human |
 |
BACKGROUND Plasminogen activator inhibitor 1 (PAI-1, Serpin E) belongs to the serine proteinase inhibitors (serpin) superfamily. PAI-1 shares important structural properties with other serpins. However, PAI-1 also exhibits unique conformational and functional properties. PAI-1 is a single-chain glycoprotein consisting of 379 or 381 amino acids (N-terminal heterogeneity) and a 23 amino acid signal peptide indicating that it is a secreted protein. It has an apparent molecular weight of 45 kDa. PAI-1 is produced by endothelial cells, smooth muscle cells, adipocytes, spleen cells, platelets, and liver cells as well as some tumor cell lines including hepatoma, melanoma, and fibrosarcoma cells. PAI-1 is the primary physiological inhibitor of uPA and tPA. It not only regulates the proteolytic activity of uPA, but also determines the level of uPA bound to uPAR by promoting the rapid endocytosis of the trimolecular uPA–PAI-1–uPAR complex. Consequently, PAI-1 plays an important role in cardiovascular diseases (mainly through inhibition of t-PA), and in cell migration and tumor development.1 At first, PAI-1 modulates cell migration by regulating ECM proteolysis. In addition, by blocking the interaction between vitronectin (VN), uPAR, and integrins, PAI-1 may induce cell detachment from the extracellular matrix and thereby promote cellular migration and tumor invasion. Furthermore, PAI-1, through its ability to titer active plasmin, promotes syndecan-1 dependent migration on unprocessed laminin-332 by preventing cleavage of the syndecan binding site LG4/5. PAI-1 inhibition of plasmin activation facilitates migration on unprocessed laminin-332 by reducing the shedding of syndecan-1 from the cell surface. Moreover, PAI-1 binding to the LRP1 in a non-uPA/uPAR dependent manner, triggers Jak/Stat1 signaling events that culminate in enhanced cell migration.2
High levels of circulating PAI-1 are associated with a number of thrombotic diseases. In animal studies, transgenic mice overexpressing human PAI-1 develop spontaneous thrombosis, whereas PAI-1-deficient mice are more resistant to venous or arterial thrombosis. Furthermore, inhibition of PAI-1 activity prevents thrombus formation in animal models. The antithrombotic effects of PAI-1 inhibition are achieved by enhancing endogenous fibrinolytic activity without directly affecting blood coagulation and platelet function. Phenotypic analysis of PAI-1 deficiency in both human and mouse suggests that inhibition of PAI-1 will not lead to severe bleeding or other major adverse effects. Thus, PAI-1 inhibitors represent a new class of antithrombotic drugs with a possible wider therapeutic index than conventional antiplatelet and anticoagulant agents.3
High levels of circulating PAI-1 are associated with a number of thrombotic diseases. In animal studies, transgenic mice overexpressing human PAI-1 develop spontaneous thrombosis, whereas PAI-1-deficient mice are more resistant to venous or arterial thrombosis. Furthermore, inhibition of PAI-1 activity prevents thrombus formation in animal models. The antithrombotic effects of PAI-1 inhibition are achieved by enhancing endogenous fibrinolytic activity without directly affecting blood coagulation and platelet function. Phenotypic analysis of PAI-1 deficiency in both human and mouse suggests that inhibition of PAI-1 will not lead to severe bleeding or other major adverse effects. Thus, PAI-1 inhibitors represent a new class of antithrombotic drugs with a possible wider therapeutic index than conventional antiplatelet and anticoagulant agents.3
REFERENCES
1. Bajou, K. et al: J. Cell Biol. 152:777-84, 2001
2. Gils, A. & Declerck, P.J. : Thromb. Haemost. 91: 425–37, 2004
3. Wilkins-Port, C.E. et al: Cell Communication Insights 3:1–10, 2010
2. Gils, A. & Declerck, P.J. : Thromb. Haemost. 91: 425–37, 2004
3. Wilkins-Port, C.E. et al: Cell Communication Insights 3:1–10, 2010
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0859 |
Target Protein Species: | Human |
Range: | 156 pg/ml – 10ng/ml |
Specificity: | No detectable cross-reactivity with other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой