PDGF-AB ELISA Kit, Rat |
 |
BACKGROUND Platelet-derived growth factor (PDGF) is a major mitogen for connective tissue cells and certain other cell types. It is a dimeric molecule consisting of disulfide-bonded, structurally similar A- and B-polypeptide chains, which combine to homo- and heterodimers. The PDGF isoforms exert their cellular effects by binding to and activating two structurally related protein tyrosine kinase receptors, denoted the alpha-receptor and the beta-receptor. The PDGF alpha-receptor binds all three isoforms with high affinity whereas the beta-receptor binds only PDGF-BB with high affinity, PDGF-AB with low affinity and does not appear to bind PDGF-AA. Ligand binding induces receptor dimerization and autophosphorylation. This enables a number of SH2 domain containing signal transduction molecules to bind to the receptors, thereby initiating various signaling pathways.1 Moreover, binding of PDGF to its receptor is followed by internalization and degradation of the ligand-receptor complex. Experiments with mutant receptors have shown that ligand-induced internalization is not absolutely dependent on the kinase activity of the beta-receptor.2
Activation of PDGF receptors leads to stimulation of cell growth, but also to changes in cell shape and motility; PDGF induces reorganization of the actin filament system and stimulates chemotaxis, i.e., a directed cell movement toward a gradient of PDGF. In vivo, PDGF isoforms have important roles during the embryonic development, particularly in the formation of connective tissue in various organs. In the adult, PDGF stimulates wound healing. Moreover, overactivity of PDGF has been implicated in several pathological conditions. The sis oncogene of simian sarcoma virus (SSV) is related to the B-chain of PDGF, and SSV transformation involves autocrine stimulation by a PDGF-like molecule. Similarly, overproduction of PDGF may be involved in autocrine and paracrine growth stimulation of human tumors. Overactivity of PDGF has, in addition, been implicated in nonmalignant conditions characterized by an increased cell proliferation, such as atherosclerosis and fibrotic conditions.3 Different kinds of PDGF antagonists are currently being developed and evaluated in different animal disease models, as well as in clinical trials.
Activation of PDGF receptors leads to stimulation of cell growth, but also to changes in cell shape and motility; PDGF induces reorganization of the actin filament system and stimulates chemotaxis, i.e., a directed cell movement toward a gradient of PDGF. In vivo, PDGF isoforms have important roles during the embryonic development, particularly in the formation of connective tissue in various organs. In the adult, PDGF stimulates wound healing. Moreover, overactivity of PDGF has been implicated in several pathological conditions. The sis oncogene of simian sarcoma virus (SSV) is related to the B-chain of PDGF, and SSV transformation involves autocrine stimulation by a PDGF-like molecule. Similarly, overproduction of PDGF may be involved in autocrine and paracrine growth stimulation of human tumors. Overactivity of PDGF has, in addition, been implicated in nonmalignant conditions characterized by an increased cell proliferation, such as atherosclerosis and fibrotic conditions.3 Different kinds of PDGF antagonists are currently being developed and evaluated in different animal disease models, as well as in clinical trials.
REFERENCES
1. Westermark, B. et al: Ciba Found Symp.150:6-14, 1990
2. Heldin, C.H. & Westermark, B.: Physiol Rev. 79:1283-316, 1999
3. Ostman, A. & Heldin, CH.: Adv Cancer Res. 80:1-38, 2001
2. Heldin, C.H. & Westermark, B.: Physiol Rev. 79:1283-316, 1999
3. Ostman, A. & Heldin, CH.: Adv Cancer Res. 80:1-38, 2001
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0485 |
Target Protein Species: | Rat |
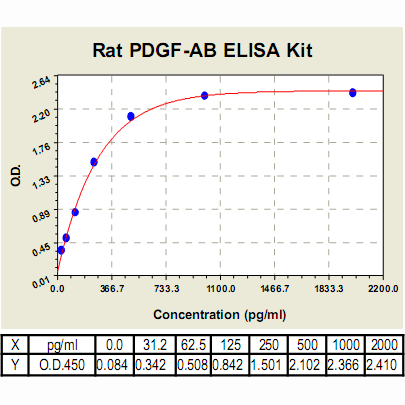
Range: | 31.2pg/ml – 2000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokine |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой