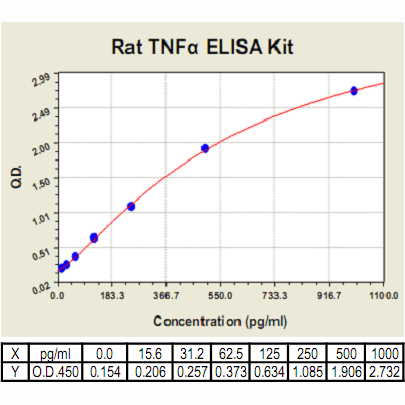
TNF-α ELISA Kit, Rat |
 |
BACKGROUND The human TNF-alpha protein contains 233 amino acids with a predicated molecular weight of 25.6 kDa. The TNF-alpha is produced initially in a membrane-associated form, which is then subjected to enzymatic remove of the N-terminal 76 amino acids by TACE/ADAM17, a TNF-alpha converting enzyme, to generate the soluble 17kDa TNF-alpha molecule that forms homotrimer. TNF-alpha is the first prototypic member identified in the TNF superfamily (TNFSF). The human TNF superfamily currently has 19 well-characterized members. Other members, such as TNFSF19, TNFSF21, and TNFSF22 have not been well-established. Although each member has its own receptor preference, a functional overlapping, such as induction of apoptosis and NF-kB activation, has been observed among the majority of these members. In addition, all of these members exhibit an evolutional conservation in their amino acid sequences, many of which show characteristics of type II membrane proteins. These features of TNF superfamily suggest that the members in this family may derived from the same ancestral gene. Several members contain a C-terminal conserved domain, named the TNF-homology domain that shares 20-30% of sequence identity. Except TNFSF1 (lymphotoxin alpha) and TNFSF3 (lymphotoxin beta) that can form either homotrimer or heterotrimer, the active form of other members in this family is homotrimer.1
TNF-alpha is expressed virtually in every type of cells in response to inflammatory signals. The most abundant cellular sources of TNF-alpha are macrophage and monocyte. In response to inflammatory stimulation, macrophage or monocyte secretes TNF-alpha that can induce apoptotic or necrotic cell death of certain tumor cell lines. In addition, TNF-alpha is also capable of inducing cell proliferation and differentiation in many types of cells under certain circumstances. TNF-alpha can be a pyrogen that causes fever by its direct action or by stimulation of interleukin 1 secretion. Sustained generation of TNF-alpha in a variety of human diseases, especially cancer and severe infection, can cause cachexia-like syndrome. The increased expression of TNF-alpha in adipose tissue was considered to be responsible for the development of obesity or diabetes due to the induction of insulin resistance by TNF-alpha.2 All of above functional characteristics of TNF-alpha are executed through specific members of the TNF receptor (TNFR) superfamily, mainly TNFR1, the primary receptor for soluble TNF-alpha, and TNFR2, the predominant receptor for membrane-associated TNF-alpha. These receptors trigger several intracellular signaling pathways, most importantly, the IkB kinase (IKK) and mitogen-activated protein kinase (MAPK) cascades, which govern gene expression through NF-kB and AP-1 transcription factors, respectively.3 TNF-alpha was found to be involved in many diseases including arthritis, asthma, cancer, cardiovascular disorders, diabetes, HIV infection and AIDS, inflammatory bowel disease, lung fibrosis, obesity, septic shock, etc. Moreover, it has been shown that mutations or polymorphisms in the promoter or coding region of TNF-alpha gene have been associated with asthma, celiac, septic shock susceptibility, silicosis, Psoriasis, GVHD, Leprosy, etc.4
TNF-alpha is expressed virtually in every type of cells in response to inflammatory signals. The most abundant cellular sources of TNF-alpha are macrophage and monocyte. In response to inflammatory stimulation, macrophage or monocyte secretes TNF-alpha that can induce apoptotic or necrotic cell death of certain tumor cell lines. In addition, TNF-alpha is also capable of inducing cell proliferation and differentiation in many types of cells under certain circumstances. TNF-alpha can be a pyrogen that causes fever by its direct action or by stimulation of interleukin 1 secretion. Sustained generation of TNF-alpha in a variety of human diseases, especially cancer and severe infection, can cause cachexia-like syndrome. The increased expression of TNF-alpha in adipose tissue was considered to be responsible for the development of obesity or diabetes due to the induction of insulin resistance by TNF-alpha.2 All of above functional characteristics of TNF-alpha are executed through specific members of the TNF receptor (TNFR) superfamily, mainly TNFR1, the primary receptor for soluble TNF-alpha, and TNFR2, the predominant receptor for membrane-associated TNF-alpha. These receptors trigger several intracellular signaling pathways, most importantly, the IkB kinase (IKK) and mitogen-activated protein kinase (MAPK) cascades, which govern gene expression through NF-kB and AP-1 transcription factors, respectively.3 TNF-alpha was found to be involved in many diseases including arthritis, asthma, cancer, cardiovascular disorders, diabetes, HIV infection and AIDS, inflammatory bowel disease, lung fibrosis, obesity, septic shock, etc. Moreover, it has been shown that mutations or polymorphisms in the promoter or coding region of TNF-alpha gene have been associated with asthma, celiac, septic shock susceptibility, silicosis, Psoriasis, GVHD, Leprosy, etc.4
REFERENCES
1. Ware, C.F.: Cytokine Growth Factor Rev. 14:181-4, 2003
2. Aggarwal,B.B. et al: Current drug targets. Inflam. and allergy 1:327-341, 2002
3. Dampsey, P.W. et al: Cytokine Growth Factor Rev. 14:193-209, 2003
4. Brennan, F.M. et al: Br J Rheumatol. 31:293-8, 1992
2. Aggarwal,B.B. et al: Current drug targets. Inflam. and allergy 1:327-341, 2002
3. Dampsey, P.W. et al: Cytokine Growth Factor Rev. 14:193-209, 2003
4. Brennan, F.M. et al: Br J Rheumatol. 31:293-8, 1992
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0526 |
Target Protein Species: | Rat |
Range: | 15.6pg/ml-1000pg/ml (Body fluids, tissue lysates or cell culture supernates) 7.8pg/ml-500pg/ml |
Specificity: | No detectable cross-reactivity with other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой