TNFRII ELISA Kit, Human |
 |
BACKGROUND Tumor necrosis factor-alpha (TNF-alpha), a proinflammatory cytokine, exerts its biological activity by signaling via its two receptors, TNFRI or p55, also classified as CD120a, and TNFRII, also referred to as p75 or CD120b. These two receptors are co-expressed in almost every cell in the body, but in contrast to the constitutive expression of TNFRI, the expression of TNFRII is strongly modulated by various cytokines including TNF-alpha, IL-1, IFN-gamma and other inflammatory stimuli like LPS. In addition, the extracellular parts of the receptors are proteolytically shed to the soluble forms by a matrix metalloproteinase-like enzyme (sTNFRI and sTNFRII). The soluble TNF receptors can neutralize TNF-alpha activities. Circulating levels of both sTNFRI and sTNFRII are elevated in patients with various diseases.1 TNFRI is generally regarded as the dominant receptor in TNF-alpha biology; it is the high affinity receptor for soluble TNF-alpha (s TNF-alpha), and mediates many of the pro-inflammatory effects of this cytokine. However, TNF effects on T cells seem to be TNFRII-mediated, as anti-TNFRII antisera or monoclonal antibodies (mAb) but not anti-TNF-RI antisera induce proliferation in the presence of a submitogenic dose of Con A.2 The absence of TNFRII significantly reduces the dermatotoxic and lethal effects of TNF-alpha. TNFRII expression is also increased on microvascular endothelial cells (ECs) in the lung during adult respiratory distress syndrome (ARDS) and is associated with the enhanced expression of cell adhesion molecules including ICAM-1, VCAM-1 and of CD14 in this context.
TNF-alpha has been shown to trigger many signaling pathways. Following oligomerization by TNF-alpha, the receptors TNFRI and TNFRII associate with adapter molecules via specific protein-protein interactions. The subsequent recruitment of downstream molecules to the receptor complex enables propagation of the TNF-alpha signal. Two cellular responses to TNF-alpha have been well documented, the induction of cell death and the activation of gene transcription for cell survival. TNF-alpha-induced apoptosis involves the activation of caspase cascades, which culminate in the cleavage of specific cellular substrates to effect cell death. TNF-alpha has also been implicated in various caspase-independent cell death processes. It was believed that TNF-induced apoptosis is mediated exclusively by TNFRI because TNFRII lacks death domain. However, it has been demonstrated that TNFRII enhances TNFRI-mediated apoptosis. Two transcription factors activated by TNF-alpha are nuclear factor kappaB (NFkappaB) and activating protein 1 (AP-1). Pathways that promote the activation of these transcription factors involve signaling molecules such as kinases, phospholipases, and sphingomyelinases. In addition to increased survival (anti-apoptotic) gene expression, NFkappaB and AP-1 also induce the expression of genes involved in inflammation, cell growth, and signal regulation.3
TNF-alpha has been shown to trigger many signaling pathways. Following oligomerization by TNF-alpha, the receptors TNFRI and TNFRII associate with adapter molecules via specific protein-protein interactions. The subsequent recruitment of downstream molecules to the receptor complex enables propagation of the TNF-alpha signal. Two cellular responses to TNF-alpha have been well documented, the induction of cell death and the activation of gene transcription for cell survival. TNF-alpha-induced apoptosis involves the activation of caspase cascades, which culminate in the cleavage of specific cellular substrates to effect cell death. TNF-alpha has also been implicated in various caspase-independent cell death processes. It was believed that TNF-induced apoptosis is mediated exclusively by TNFRI because TNFRII lacks death domain. However, it has been demonstrated that TNFRII enhances TNFRI-mediated apoptosis. Two transcription factors activated by TNF-alpha are nuclear factor kappaB (NFkappaB) and activating protein 1 (AP-1). Pathways that promote the activation of these transcription factors involve signaling molecules such as kinases, phospholipases, and sphingomyelinases. In addition to increased survival (anti-apoptotic) gene expression, NFkappaB and AP-1 also induce the expression of genes involved in inflammation, cell growth, and signal regulation.3
REFERENCES
1. Nozaki, N. et al: J. Mol. Cell Cardiol. 30:2003-12, 1998
2. Aspalter, R.M. et al: J. Leuk. Biol. 74:572-82, 2003
3. Leong, K.G. & Karsan, A.: Histol Histopathol. 15:1303-25, 2000
2. Aspalter, R.M. et al: J. Leuk. Biol. 74:572-82, 2003
3. Leong, K.G. & Karsan, A.: Histol Histopathol. 15:1303-25, 2000
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0530 |
Target Protein Species: | Human |
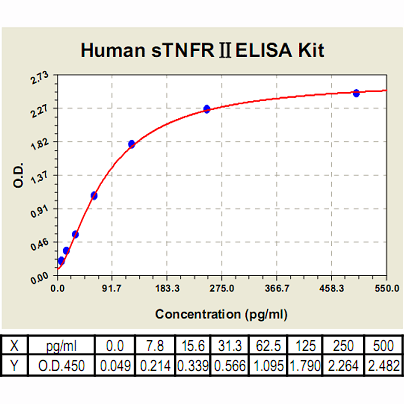
Range: | 7.8 pg/ml – 500pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой