uPA ELISA Kit, Human |
 |
BACKGROUND Urokinase-type plasminogen activator (uPA) is a highly restricted serine protease. The primary physiological substrate is plasminogen, which is an inactive zymogen form of the serine protease plasmin. Activation of plasmin triggers a proteolysis cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This links urokinase to vascular diseases and cancer. uPA has also been shown to induce adhesion and chemotactic movement of myeloid cells, to induce cell migration in human epithelial cells and bovine endothelial cells, and to promote cell growth. These signaling functions of uPA do not require its proteolytic activity.1
Urokinase is a 411-residue protein, consisting of three domains: growth factor domain (GFD) (residue 1–43), kringle domain (residue 50–131), and serine protease domain (residue 159–411). Urokinase is synthesized as a zymogen form (prourokinase or single-chain urokinase), and is activated by proteolytic cleavage between L158 and I159. The two resulting chains are kept together by a disulfide bond. uPA binds with high affinity through GFD to a cell-surface receptor (uPAR/CD87) that has been identified in many cell types. uPAR is a glycosylphosphatidylinositol-anchored 35–55-kDa glycoprotein. It is generally accepted that uPA-mediated signaling requires prior binding to uPAR.2 It was shown that uPA stimulated-migration of human vascular smooth muscle cells was involved in TYK2 and phosphatidylinositol 3-kinase (PI3-K) pathways.3
The most important inhibitors of urokinase are the serpins plasminogen activator inhibitor-1 (PAI-1) and plasminogen activator inhibitor-2 (PAI-2), which inhibit the protease activity irreversibly. Elevated expression levels of urokinase and several other components of the plasminogen activation system are found to be correlated with tumor malignancy. It is believed that the tissue degradation following plasminogen activation facilitates tissue invasion and, thus, contributes to metastasis. This makes urokinase an attractive drug target, and, so, inhibitors have been sought to be used as anticancer agents. Through its interaction with the urokinase receptor, urokinase affects several other aspects of cancer biology such as cells adhesion, migration, and cellular mitotic pathways. Urokinase is used clinically as a thrombolytic agent in the treatment of severe or massive deep venous thrombosis, pulmonary embolism, myocardial infarction, and occluded intravenous or dialysis cannulas. It is also administered intrapleurally to improve the drainage of complicated pleural effusions and empyemas.4
Urokinase is a 411-residue protein, consisting of three domains: growth factor domain (GFD) (residue 1–43), kringle domain (residue 50–131), and serine protease domain (residue 159–411). Urokinase is synthesized as a zymogen form (prourokinase or single-chain urokinase), and is activated by proteolytic cleavage between L158 and I159. The two resulting chains are kept together by a disulfide bond. uPA binds with high affinity through GFD to a cell-surface receptor (uPAR/CD87) that has been identified in many cell types. uPAR is a glycosylphosphatidylinositol-anchored 35–55-kDa glycoprotein. It is generally accepted that uPA-mediated signaling requires prior binding to uPAR.2 It was shown that uPA stimulated-migration of human vascular smooth muscle cells was involved in TYK2 and phosphatidylinositol 3-kinase (PI3-K) pathways.3
The most important inhibitors of urokinase are the serpins plasminogen activator inhibitor-1 (PAI-1) and plasminogen activator inhibitor-2 (PAI-2), which inhibit the protease activity irreversibly. Elevated expression levels of urokinase and several other components of the plasminogen activation system are found to be correlated with tumor malignancy. It is believed that the tissue degradation following plasminogen activation facilitates tissue invasion and, thus, contributes to metastasis. This makes urokinase an attractive drug target, and, so, inhibitors have been sought to be used as anticancer agents. Through its interaction with the urokinase receptor, urokinase affects several other aspects of cancer biology such as cells adhesion, migration, and cellular mitotic pathways. Urokinase is used clinically as a thrombolytic agent in the treatment of severe or massive deep venous thrombosis, pulmonary embolism, myocardial infarction, and occluded intravenous or dialysis cannulas. It is also administered intrapleurally to improve the drainage of complicated pleural effusions and empyemas.4
REFERENCES
1. Finckh, U. et al.: Neurogenetics 4: 213-217, 2003
2. Tarui, T. et al: J. Biol. Chem. 278:29863-78, 2003
3. Kiian, I. et al.: Thromb. Haemost. 89: 904-914, 2003
4. Harbeck, N. et al: Thromb Haemost. 91:450-6, 2004
2. Tarui, T. et al: J. Biol. Chem. 278:29863-78, 2003
3. Kiian, I. et al.: Thromb. Haemost. 89: 904-914, 2003
4. Harbeck, N. et al: Thromb Haemost. 91:450-6, 2004
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CL0535 |
Target Protein Species: | Human |
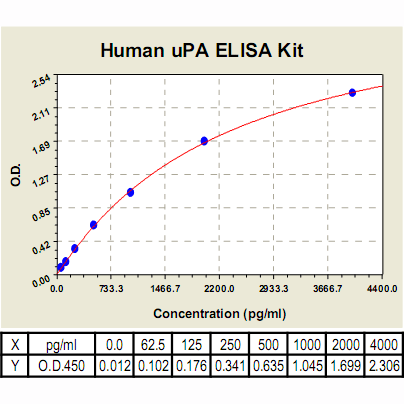
Range: | 62.5 pg/ml - 4000 pg/ml |
Specificity: | No detectable cross-reactivity with other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой