Anti-AdoMetDC/SAMDC: Rabbit S-Adenosylmethionine Decarboxylase Antibody |
 |
BACKGROUND S-Adenosylmethionine decarboxylase (AdoMetDC/SAMDC, EC4.1.1.50) is an essential enzyme for the biosynthesis of the polyamines spermidine and spermine, which are required for normal cell proliferation and differentiation. AdoMetDC/SAMDC catalyzes the removal of the carboxyl group from S-adenosylmethionine (AdoMet) to form decarboxylated S-adenosylmethionine (deAdoMet). The propylamine group of deAdo-Met is transferred to putrescine to form spermidine or from spermidine to form spermine. AdoMetDC/SAMDC is one member of a small class of decarboxylases that use a covalently bound pyruvate as a prosthetic group. All known AdoMetDC/SAMDC are synthesized in the proenzyme form which subsequently undergoes an intramolecular cleavage at a serine residue to generate two subunits. In this process, the serine residue is converted to a pyruvoyl group. Proenzyme processing generates two non-identical subunits, termed A and B, which are both indispensable components of the mature enzyme. The first step of AdoMetDC/SAMDC catalysis is AdoMet binding through the pyruvate prosthetic group, which reacts to form a Schiff base adducts with the substrate. It is postulated that Cys82 is the proton donor in the decarboxylation reaction catalyzed by AdoMetDC/SAMDC. The importance of Cys82 is illustrated by the fact that mutation of this residue to serine or alanine greatly reduces the enzyme’s activity.1
AdoMetDC/SAMDC is very highly regulated at a number of levels including transcription, translation, proenzyme processing, catalytic activity and degradation. Elevated polyamine (PA) levels are sustained in rapidly proliferating cells and both AdoMetDC/SAMDC and PA levels are often up-regulated in cancer cells. Depletion of polyamine content in colorectal cancer by chemotherapy is related to tumor regression and impaired tumorigenicity. It was demonstrated that elevations in SAMDC expression were induced by H-ras transformation in metastatic C3 cells. Moreover, PKC and cAMP-mediated signaling pathways play important role in the regulation of SAMDC expression in highly malignant C3 cells. Furthermore, it was shown that growth factors including EGF, bFGF also induced alterations in SAMDC expression, which is involved in the promotion and establishment of events important to the phenotype expressed by H-ras transformed cells capable of malignant progression.2 Thus, inhibition of AdoMetDC/SAMDC provides an attractive therapeutic target for many cancers. It was shown that Inhibition of AdoMetDC/SAMDC by inhibitor SAM486A connects polyamine metabolism with p53-Mdm2-Akt/PKB regulation and apoptosis in neuroblastoma.3 Different AdoMetDC/SAMDC inhibitors are in anti-cancer clinical trials.
AdoMetDC/SAMDC is very highly regulated at a number of levels including transcription, translation, proenzyme processing, catalytic activity and degradation. Elevated polyamine (PA) levels are sustained in rapidly proliferating cells and both AdoMetDC/SAMDC and PA levels are often up-regulated in cancer cells. Depletion of polyamine content in colorectal cancer by chemotherapy is related to tumor regression and impaired tumorigenicity. It was demonstrated that elevations in SAMDC expression were induced by H-ras transformation in metastatic C3 cells. Moreover, PKC and cAMP-mediated signaling pathways play important role in the regulation of SAMDC expression in highly malignant C3 cells. Furthermore, it was shown that growth factors including EGF, bFGF also induced alterations in SAMDC expression, which is involved in the promotion and establishment of events important to the phenotype expressed by H-ras transformed cells capable of malignant progression.2 Thus, inhibition of AdoMetDC/SAMDC provides an attractive therapeutic target for many cancers. It was shown that Inhibition of AdoMetDC/SAMDC by inhibitor SAM486A connects polyamine metabolism with p53-Mdm2-Akt/PKB regulation and apoptosis in neuroblastoma.3 Different AdoMetDC/SAMDC inhibitors are in anti-cancer clinical trials.
REFERENCES
1. WADA, M. & SHIRAHATA, A.: Biol. Pharm. Bull. 33: 891-4, 2010
2. Hardin, M.S. & Hurta, R.A.: J. Cell. Biochem. 84:349-58, 2002
3. Koomoa, D-L.T. et al: Mol. Cancer Ther. 8:2067-75, 2009
2. Hardin, M.S. & Hurta, R.A.: J. Cell. Biochem. 84:349-58, 2002
3. Koomoa, D-L.T. et al: Mol. Cancer Ther. 8:2067-75, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1070 |
Antigen: | Short peptide from human AdoMetDC/SAMDC sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
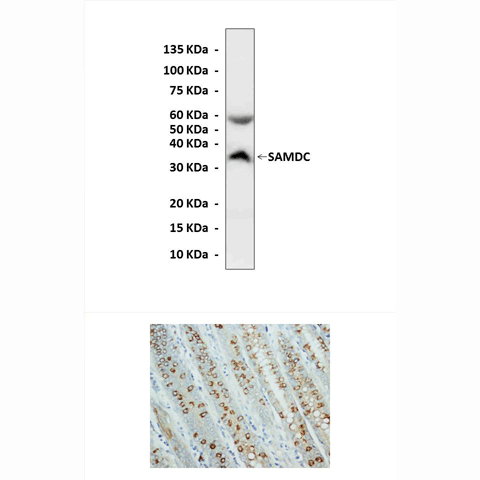
Predicted Molecular Weight of protein: | 35 kDa |
Specificity/Sensitivity | Detects endogenous levels of AdoMetDC/SAMDC proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой