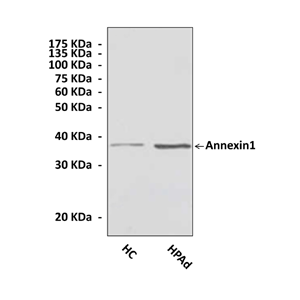
Anti-Annexin1: Polyclonal Annexin1 Antibody |
 |
BACKGROUND Annexins are highly conserved calcium- and phospholipid-binding proteins that share common structures but exert diverse functions. Annexins have been variously implicated in cell differentiation and proliferation, extracellular processes such as coagulation, and membrane fusion events including endocytosis and exocytosis. The common calcium- and phospholipid-binding activities of annexins reside in the highly conserved core domain, whereas the unique properties of each annexin are mediated by divergent N termini.1 Complex interactions of annexins with cellular components such as membranes, cytoskeletal elements, and subcellular signaling assemblies result in multiple effects.
Annexin1 (previously lipocortin-1) is a potent inhibitor of inflammation both in vitro and in vivo. Induced in some cell types in response to glucocorticoids, annexin-1 was initially proposed to act intracellularly to inhibit cytosolic phospholipase A2, reducing prostaglandin biosynthesis. It can also affect many other components of the inflammatory reaction including Cox2 and iNOS.2 Studies indicate that annexin-1 can also be secreted , and participates in additional anti-inflammatory effects including detachment of neutrophils from vascular endothelium. The effects of secreted annexin-1 appear to derive from engagement of specific cell surface receptors by both the intact secreted protein and by various N-terminal-derived peptide fragments. In contrast to its better-appreciated anti-inflammatory effects, extracellular annexin-1 has been shown to exert a prosecretory role in some cell types through as yet uncharacterized receptors and to enhance chemotaxis and cellular polarization in neutrophils by its actions on members of the formyl peptide receptor (FPR) family.3 It was demonstrated that annexin-1 autoengaged FPRL-1 to stimulate Erk, Jnk, and NF-kB activation, resulting in MMP-1 secretion in TNF-alpha-treated cells. It was shown recently that annexin-1 not only ANXA1 promoted inflammatory cell apoptosis associated with transient rise in intracellular calcium and caspase-3 activation, but also was identified as one of the 'eat-me' signals on apoptotic cells to be recognised and ingested by phagocytes.4
REFERENCES
1. Ciocca DR et al.: J. Natl Can. Inst., 85: 1558-1570, 1993.
2. Landry J et al.: J. Biol. Chem. 267: 794-803, 1992.
3. Ciocca DR & Calderwood SK: Cell Stress Chaperones, 10:86-103, 2005.
2. Landry J et al.: J. Biol. Chem. 267: 794-803, 1992.
3. Ciocca DR & Calderwood SK: Cell Stress Chaperones, 10:86-103, 2005.
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1006 |
Antigen: | Human Annexin1 N-terminal sequence. |
Isotype: | Rabbit Polyclonal |
Species & predicted species cross- reactivity ( ): | Human, Rat, Mouse |
Applications & Suggested starting dilutions: | WB 1:1000 IP 1:50 IHC (Paraffin) 1:100 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 37 kDa |
Specificity/Sensitivity: | Detects endogenous levels of Annexin1 protein in normal primary cell lysates. |
Storage: | Store at 4° C for frequent use; at -20° C for at least one year. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой