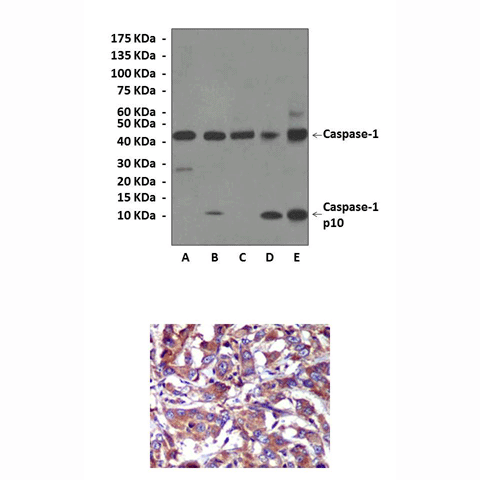
Anti-Caspase-1: Rabbit Caspase-1/ICE Antibody |
 |
BACKGROUND Caspases, short for cysteinyl aspartate proteases, are involved in the signal transduction pathways of apoptosis, necrosis and inflammation. These enzymes can be divided into two major classes: initiators and effectors. The initiator isoforms (Caspases-1,-4,-5,-8,-9,-10,-11,-12) are activated by, and interact with, upstream adaptor molecules through protein-protein interaction domains known as CARD and DED. Effector Caspases (-3,-6,-7) are responsible for cleaving downstream substrates and are sometimes referred to as the executioner Caspases. More than 400 Caspase substrates have so far been identified. Initiator Caspases, such as Caspase 8, may be directly activated by death receptors such as FasR. Caspases can also be found intracellularly as part of large multiprotein complexes. For example, Caspase 9 is recruited to the apoptosome formed during apoptosis, while Caspases-1 and 5 can form part of the inflammasome, a key part of cytokine processing during inflammation. Caspases are regulated by inhibitors of apoptosis and by dominant negative isoforms. They have been implicated in the pathogenesis of many disorders including stroke, Alzheimer's disease, myocardial infarction, cancer, and inflammatory disease.1
ProCaspase-1 also contains an NH2-terminal CARD domain, suggesting that its mechanism of activation, like that of proCaspase-9, involves association with an Apaf-1-related molecule.2 Caspase-1 is, also called interleukin-1beta converting enzyme (ICE/ICEalpha), a thiol protease that cleaves IL-1 beta between an Asp and an Ala, releasing the mature cytokine which is involved in a variety of inflammatory processes. In addition, IL-18 is also substrate of Caspase-1. Caspase-1 has been shown to induce cell apoptosis and may function in various developmental stages. Studies of a similar gene in mouse suggest a role in the pathogenesis of Huntington disease. It plays important role in host defense against pathogens. It also cleaves and activates sterol regulatory element binding proteins (SREBPs). Alternative splicing of Caspase-1 gene results in five transcript variants encoding distinct isoforms.3
ProCaspase-1 also contains an NH2-terminal CARD domain, suggesting that its mechanism of activation, like that of proCaspase-9, involves association with an Apaf-1-related molecule.2 Caspase-1 is, also called interleukin-1beta converting enzyme (ICE/ICEalpha), a thiol protease that cleaves IL-1 beta between an Asp and an Ala, releasing the mature cytokine which is involved in a variety of inflammatory processes. In addition, IL-18 is also substrate of Caspase-1. Caspase-1 has been shown to induce cell apoptosis and may function in various developmental stages. Studies of a similar gene in mouse suggest a role in the pathogenesis of Huntington disease. It plays important role in host defense against pathogens. It also cleaves and activates sterol regulatory element binding proteins (SREBPs). Alternative splicing of Caspase-1 gene results in five transcript variants encoding distinct isoforms.3
REFERENCES
1. Riedl, S.J. & Shi, Y. : Nature Rev. Mol. Cell Biol. 5:897-907, 2004
2. Poyet, J. et al: J. Biol. Chem. 276:28309-13, 2001
3. Fantuzzi, G. & Dinarello, C.A.:J. Clin. Immunol. 19:1-11, 1999
2. Poyet, J. et al: J. Biol. Chem. 276:28309-13, 2001
3. Fantuzzi, G. & Dinarello, C.A.:J. Clin. Immunol. 19:1-11, 1999
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
|
Cat.No.:
|
CG1697
|
|
Antigen:
|
Short peptide from the C-terminal sequence of human pro-Caspase-1.
|
|
Isotype:
|
Rabbit IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human, Mouse, Rat
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:1000 - 1:10000
IP n/d
IHC 1:250 - 1:500
ICC 1:100 - 1:250
FACS n/d
|
|
Predicted Molecular
Weight of protein:
|
45/10 kDa
|
|
Specificity/Sensitivity:
|
Detects endogenous Caspase-1 proteins without cross-reactivity with other family proteins.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой