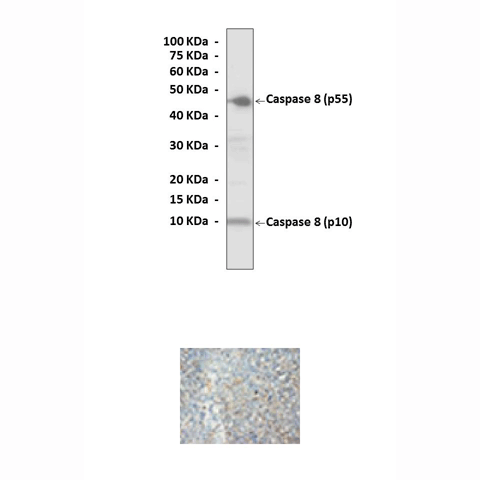
Anti-Caspase-8: Polyclonal Caspase-8 Antibody |
 |
BACKGROUND Caspases, short for cysteinyl aspartate proteases, are involved in the signal transduction pathways of apoptosis, necrosis and inflammation. These enzymes can be divided into two major classes: initiators and effectors. The initiator isoforms (Caspases-1,-4,-5,-8,-9,-10,-11,-12) are activated by, and interact with, upstream adaptor molecules through protein-protein interaction domains known as CARD and DED. Effector Caspases (-3,-6,-7) are responsible for cleaving downstream substrates and are sometimes referred to as the executioner Caspases. More than 400 Caspase substrates have so far been identified. Initiator Caspases, such as Caspase 8, may be directly activated by death receptors such as FasR. Caspases can also be found intracellularly as part of large multiprotein complexes. For example, Caspase 9 is recruited to the apoptosome formed during apoptosis, whilst Caspases-1 and 5 can form part of the inflammasome, a key part of cytokine processing during inflammation. Caspases are regulated by inhibitors of apoptosis and by dominant negative isoforms. They have been implicated in the pathogenesis of many disorders including stroke, Alzheimer's disease, myocardial infarction, cancer, and inflammatory disease.1
Caspases exist as inactive proenzymes which undergo proteolytic processing at conserved aspartic residues to produce two subunits, large and small, that dimerize to form the active enzyme. Sequential activation of Caspases plays a central role in the execution-phase of cell apoptosis.2 Most upstream protease of the activation cascade of Caspases responsible for the TNFRSF6/FAS mediated and TNFRSF1A induced cell death. The N-terminal FADD-like death effector domain of Caspase-8 interacts with Fas-interacting protein FADD, which recruits it to either receptor. The resulting aggregate called death-inducing signaling complex (DISC) performs Caspase-8 proteolytic activation.3 The active dimeric enzyme is then liberated from the DISC and free to activate downstream apoptotic proteases. Proteolytic fragments of the N-terminal propeptide (termed CAP3, CAP5 and CAP6) are likely retained in the DISC. It cleaves and activates CASP3, CASP4, CASP6, CASP7, CASP9 and CASP10.
Caspases exist as inactive proenzymes which undergo proteolytic processing at conserved aspartic residues to produce two subunits, large and small, that dimerize to form the active enzyme. Sequential activation of Caspases plays a central role in the execution-phase of cell apoptosis.2 Most upstream protease of the activation cascade of Caspases responsible for the TNFRSF6/FAS mediated and TNFRSF1A induced cell death. The N-terminal FADD-like death effector domain of Caspase-8 interacts with Fas-interacting protein FADD, which recruits it to either receptor. The resulting aggregate called death-inducing signaling complex (DISC) performs Caspase-8 proteolytic activation.3 The active dimeric enzyme is then liberated from the DISC and free to activate downstream apoptotic proteases. Proteolytic fragments of the N-terminal propeptide (termed CAP3, CAP5 and CAP6) are likely retained in the DISC. It cleaves and activates CASP3, CASP4, CASP6, CASP7, CASP9 and CASP10.
REFERENCES
1. Riedl, S.J. & Shi, Y.: Nature Rev. Mol. Cell Biol. 5:897-907, 2004
2. Nickolson, D.W. et al: Nature 367:37-43, 1995
3. Bidere, N. et al: Curr. Biol. 16:1666-71, 2006
2. Nickolson, D.W. et al: Nature 367:37-43, 1995
3. Bidere, N. et al: Curr. Biol. 16:1666-71, 2006
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA0689 |
Antigen: | N-terminal sequence of human caspase-8 p10 subunit |
Isotype: | Affinity-purified Rabbit polyclonal IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat, Rabbit |
Applications & Suggested starting dilutions: | WB 1:500 to 1:1000 IP n/d IHC (Paraffin) 1:50 to 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 55 kDa & 10 kDa |
Specificity/Sensitivity: | Reacts specifically with Caspase-8 of human, rabbit, mouse & rat origin in Immunohistochemical staining and western blotting, no cross-reactivity with other members of the family. |
Storage: | Store at 4° C for frequent use; at -20° C for at least one year. |
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой