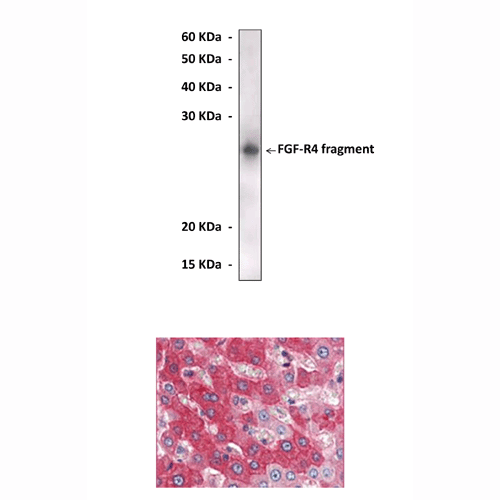
Anti-FGF-R4: Mouse Fibroblast Growth Factor Receptor Tyrosine Kinase 4 Antibody |
 |
BACKGROUND Fibroblast Growth Factors (FGFs) and their receptors constitute an elaborate signaling system that participates in many developmental and repair processes of virtually all mammalian tissues. Among the 23 FGF members, ten have been identified in the brain. Four FGF receptor tyrosine kinases (FGFR1-4) are known so far.1 Ligand binding of these receptors greatly depends on the presence of heparan sulfate proteoglycans, which act as low affinity FGFRs. Ligand binding specificity of FGFRs depends on the third extracellular Ig-like domain, which is subject to alternative splicing. The FGF elicits the regulatory activity by binding to FGF receptor (FGFR)-heparin sulfate complexes and inducing receptor auto-phosphorylation, as well as phosphorylation of downstream signaling molecules. These include phosphorylation of Src and PLC-gamma, leading finally to activation of PKC, Crk and Shc.
SNT/FRS2 serves as an alternative link between FGFRs to the activation of PKC, and additionally activates the Ras signaling cascade.2 Deregulation of FGFR signaling, by activating mutations or ligand/receptor overexpression, could allow these receptors to become constitutively active, leading to cancer development. These cancers include hematopoietic and solid tumors (breast, bladder, and prostate carcinomas).
SNT/FRS2 serves as an alternative link between FGFRs to the activation of PKC, and additionally activates the Ras signaling cascade.2 Deregulation of FGFR signaling, by activating mutations or ligand/receptor overexpression, could allow these receptors to become constitutively active, leading to cancer development. These cancers include hematopoietic and solid tumors (breast, bladder, and prostate carcinomas).
The FGFR-4 gene is located on chromosome 13 in mice and 5q33-qter in humans, and is unusual in that it does not undergo the alternative splicing described for the other receptors. FGFR-4 binds strongly to acidic FGF, basic FGF and FGF-4, FGF-6, FGF-8, FGF-9, FGF15, and FGF19 and it also binds FGF-3 and FGF-5 with less affinity. FGFR-4 associates with an 85 kDa serine kinase, which it can activate in a process that is dependent on ligand binding but independent of receptor tyrosine phosphorylation. FGFR4 plays an important role in development. During mouse embryonic development, FGFR4 expression was found early in the endodermal compartments of the gut and yolk sac and in the myotomal compartment of the somites, then in the developing skeletal muscles, a number of endodermally derived tissues including liver, lung and pancreas, and the adrenal cortex and kidney. FGFR4 is also found in the ventricular zone and cortex of the murine brain. FGFR4 expression has also been seen in a number of mammary carcinomas, suggesting an involvement in breast cancer.3 On the other hand, FGFR4 was shown to be involved in regulation of metabolism. FGFR4 may a primary target of insulin regulation in hepatocytes. Both insulin and FGF activate the PI 3-kinase pathway and FoxO1 is a key node in the convergence of FGF and insulin signaling pathways and functions as a key integrator for the regulation of glucose and bile acid metabolism.4
REFERENCES
1. Merkel, M. Et al: J. Biol. Chem. 277:7405-11, 2001
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Kamei, Y. et al: Biochem. Int. 26:923-34, 1992
2. Hamilton, M.T. et al: Am J Physiol Endocrinol Metab 275: E1016-E1022, 1998
3. Kamei, Y. et al: Biochem. Int. 26:923-34, 1992
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10103 |
Antigen: | Purified recombinant human FGFR4 fragments expressed in E. coli. |
Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 120 kDa |
Specificity/Sensitivity: | Detects FGFR4 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
|
| ||||||||||
| ||||||||||
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой