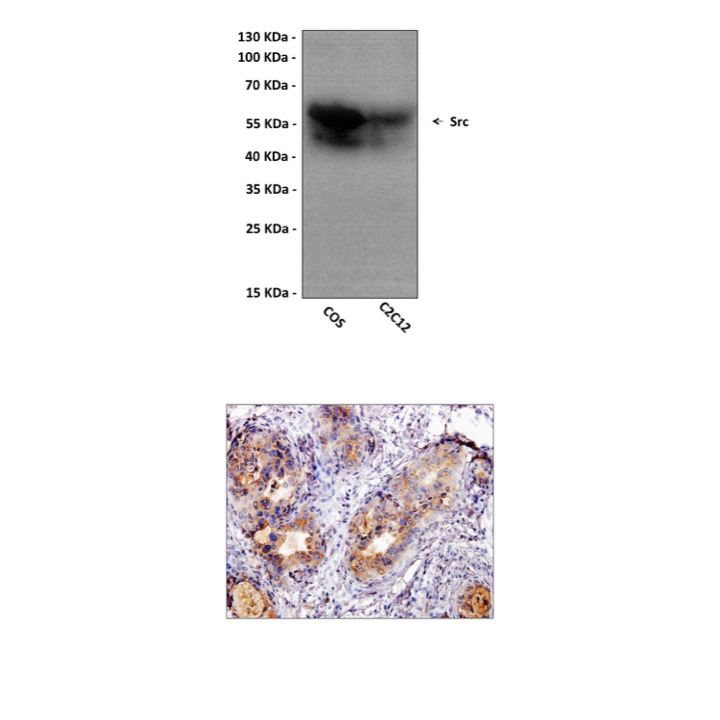
Anti-Src: Mouse Src Antibody |
 |
BACKGROUND Src was the first proto-oncogene described, and was among the first molecules in which tyrosine kinase activity was documented. The Src gene family has nine known members (Blk, c-Fgr, Fyn, Hck, Lck, Lyn, c-Src, c-Yes and Yrk), each encoding a cytoplasmic protein-tyrosine kinase (PTK) believed to be involved in signal transduction. The Src PTKs contain three domains (SH1, SH2 and SH3) that are found in many other signaling proteins. The SH1 domain has PTK activity, whilst the SH2 and SH3 domains are involved in mediating protein-protein interactions by binding to phosphor-tyrosine-containing and proline rich motifs, respectively. The expression patterns of the c-Src PTKs suggest that they function in a broad range of biological situations, in many cases regulating the behavior of terminally differentiated, post-mitotic cell types (1). Src has been defined as a common modular structure that participates in much of the crosstalk between the cytoplasmic protein tyrosine kinases and tyrosine kinase receptors (2). Src has been found to be overexpressed and activated in a large number of different cancers. Cellular Src has been shown to activate a number of different effectors that are involved in different aspects of cancer biology, such as metastasis, cell cycle regulation and cell survival (3, 4). Cumulative data marks Src signaling as attractive therapeutic targets in different cancers.
REFERENCES
1. Homsi J et al., Expert Opin Ther Targets. 11:91-100, 2007.
2. Brickell PM et al., Crit Rev Oncog 3:401, 1993.
3. Bolen JB et al., FASEB J 6:3403, 1992.
4. Alvarez RH et al., Cancer 107:1918, 2006.
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
| Cat.No.: | CC10054 |
| Antigen: | Recombinant human c-Src expressed in Sf9 cells. |
| Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat, Monkey |
Applications & Suggested starting dilutions:* | WB 1:500 IP n/d IHC 1:100 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 60 kDa |
| Specificity/Sensitivity: | Detects endogenous Src proteins without cross-reactivity with other family members. |
| Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой