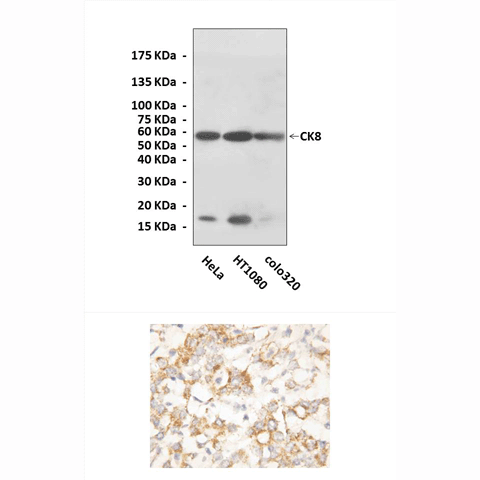
Anti-CK8: Rabbit Cytokeratin 8 Antibody |
 |
BACKGROUND Cytokeratins (CKs) are the largest class of cytoskeletal intermediate filament proteins. There are 20 different Cytokeratin proteins (assigned numbers 1-20), which are structural markers specific for epithelial cells, and are expressed specifically in different types of epithelial tissue. Cytokeratins are primarily insoluble molecules that play an important role in cellular mechanics, including: cell shape, motility, division and cell-cell contact.
There are two types of Cytokeratins. Type I (9–20) Cytokeratins are relatively acidic and are of small molecular weight (40–56.5 kDa). Type II (1–8) Cytokeratins are relatively basic-neutral and of larger molecular weight (53–67 kDa). Expression of CK in epithelia is dependent on types (simple or stratified), and differentiation pattern of epithelia. CK can be subdivided into markers of cornification (CK 1, 2, 10, and 11), stratification (CK4, 13), basal cells (CK 5, 14, 15, and 17), simple cells (CK 7, 8, 18, and 19), and hyperproliferation (CK6, 16). Although CKs are expressed in specific combinations in different epithelia, the number of permutations is limited, and thus insufficient to endow every epithelium with its own unique CK profile.1
Cytokeratin 18 is expressed at high levels by many cells found in single layer epithelial tissues. This protein, together with cytokeratin 8, exhibits resistance features in response to stress and to apoptosis. It is often used together with cytokeratin 8 and CK19 to differentiate cells of epithelial origin from hematopoietic cells in tests that enumerate circulating tumor cells in blood.2 Cytokeratin 8 associates with CK18 to form an insoluble matrix within the cell. The CK8/CK18 complex modulates the signaling pathways intracellularly by binding kinases involved in signal transduction. It integrates signals generated by stimulated surface membrane receptors, such as insulin receptor and integrin beta 1 receptor, and modulates their signal transduction in appropriate reaction sequences. The absence of CK8/CK18 in hepatocytes results in a phenotype that attaches more rapidly to fibronectin and undergoes an enhanced G1/S transition compared with wild-type hepatocytes. On the other hand, the cytoskeleton itself can undergo rearrangements as a result of outside-in signals triggered by fibronectin binding to cell surface receptors, the integrins. Cytokeratin 8 is unique among cytokeratins in that it contains a carboxyl-terminal lysine that can interact with the lysine binding sites of plasminogen. It was demonstrated that cytokeratin 8, together with uPA, plasminogen and fibronectin, constitutes a signaling platform capable of modulating cell adhesion/growth-dependent signal transduction in breast tumor cells. Anti-cytokeratin 8 mAb, which competes for the binding site for uPA, could be used as an agent to reduce the invasive potential of breast tumor cells.3
There are two types of Cytokeratins. Type I (9–20) Cytokeratins are relatively acidic and are of small molecular weight (40–56.5 kDa). Type II (1–8) Cytokeratins are relatively basic-neutral and of larger molecular weight (53–67 kDa). Expression of CK in epithelia is dependent on types (simple or stratified), and differentiation pattern of epithelia. CK can be subdivided into markers of cornification (CK 1, 2, 10, and 11), stratification (CK4, 13), basal cells (CK 5, 14, 15, and 17), simple cells (CK 7, 8, 18, and 19), and hyperproliferation (CK6, 16). Although CKs are expressed in specific combinations in different epithelia, the number of permutations is limited, and thus insufficient to endow every epithelium with its own unique CK profile.1
Cytokeratin 18 is expressed at high levels by many cells found in single layer epithelial tissues. This protein, together with cytokeratin 8, exhibits resistance features in response to stress and to apoptosis. It is often used together with cytokeratin 8 and CK19 to differentiate cells of epithelial origin from hematopoietic cells in tests that enumerate circulating tumor cells in blood.2 Cytokeratin 8 associates with CK18 to form an insoluble matrix within the cell. The CK8/CK18 complex modulates the signaling pathways intracellularly by binding kinases involved in signal transduction. It integrates signals generated by stimulated surface membrane receptors, such as insulin receptor and integrin beta 1 receptor, and modulates their signal transduction in appropriate reaction sequences. The absence of CK8/CK18 in hepatocytes results in a phenotype that attaches more rapidly to fibronectin and undergoes an enhanced G1/S transition compared with wild-type hepatocytes. On the other hand, the cytoskeleton itself can undergo rearrangements as a result of outside-in signals triggered by fibronectin binding to cell surface receptors, the integrins. Cytokeratin 8 is unique among cytokeratins in that it contains a carboxyl-terminal lysine that can interact with the lysine binding sites of plasminogen. It was demonstrated that cytokeratin 8, together with uPA, plasminogen and fibronectin, constitutes a signaling platform capable of modulating cell adhesion/growth-dependent signal transduction in breast tumor cells. Anti-cytokeratin 8 mAb, which competes for the binding site for uPA, could be used as an agent to reduce the invasive potential of breast tumor cells.3
REFERENCES
1. Makino T et al.: Br. J. Cancer 101:1298-1306 (2009)
2. Allard WJ et al.: Clin. Cancer Res. 10:6897-6904 (2004)
3. Obermajer, N. et al: Mol Cancer. 21;8:88, 2009
2. Allard WJ et al.: Clin. Cancer Res. 10:6897-6904 (2004)
3. Obermajer, N. et al: Mol Cancer. 21;8:88, 2009
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1240 |
Antigen: | Short peptide from human CK8 sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 56 kDa |
Specificity/Sensitivity: | Detects endogenous levels of CK8 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой