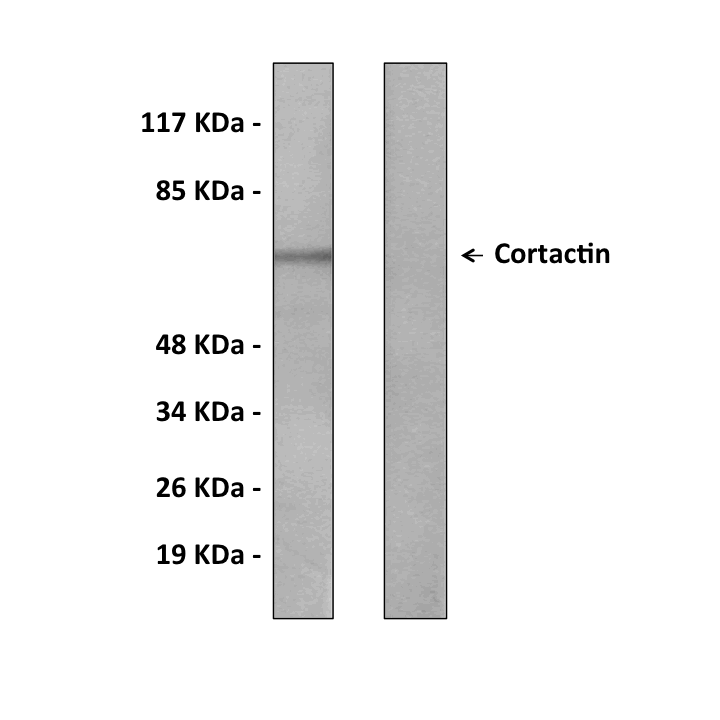
Anti-Cortactin: Rabbit Cortactin Antibody |
 |
BACKGROUND Cortactin was first identified as both an F-actin binding protein and v-Src substrate and was suggested to be a key regulator of actin rearrangements in response to tyrosine kinase signaling. The recent discovery that Cortactin binds and activates the actin related protein (Arp)2/3 complex, and thus regulates the formation of branched actin networks, together with the identification of multiple protein targets of the Cortactin SH3 domain, have revealed diverse cellular roles for this protein. It is now clear that the modular nature of Cortactin enables coupling of two key biochemical properties, the ability to nucleate and stabilize Arp2/3-generated dendritic actin networks and SH3 domain-mediated targeting to specific protein complexes.1 Cortactin is localized in the cytoplasm and in areas of the cell-substratum contacts. It has two roles: (1) regulating the interactions between components of adherens-type junctions and (2) organizing the cytoskeleton and cell adhesion structures of epithelia and carcinoma cells. During apoptosis, Cortactin is degraded in a caspase-dependent manner. The aberrant regulation of this protein contributes to tumor cell invasion and metastasis. Cortactin was found to be overexpressed in breast cancer and squamous cell carcinomas of the head and neck. Src phosphorylates murine cortactin in vitro on three sites (Tyr421, Tyr466 and Tyr482). In addition, Cortactin has two consensus ERK phosphorylation sites (Ser405 and Ser418).2 Studies on thrombin-induced lamellipodial spreading in platelets have implicated a second serine/threonine kinase in Cortactin regulation, the Rac/Cdc42 effector PAK (p21-activated kinase).3
REFERENCES
1. Daly, R.J.: Biochem. J. 382:13-25, 2004
2. Ayala, I. et al: J. Cell Sci. 121:369-78, 2008
3. Vidal, C. et al: Blood 100:4462-69, 2002
2. Ayala, I. et al: J. Cell Sci. 121:369-78, 2008
3. Vidal, C. et al: Blood 100:4462-69, 2002
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1094 |
Antigen: | Synthesized peptide derived from human Cortactin |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:500-1:1000 IP n/d IHC n/d ICC n/d FACS n/d ELISA 1:20000 |
Predicted Molecular Weight of protein: | 61 KDa |
Specificity/Sensitivity: | Detects endogenous Cortactin proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой