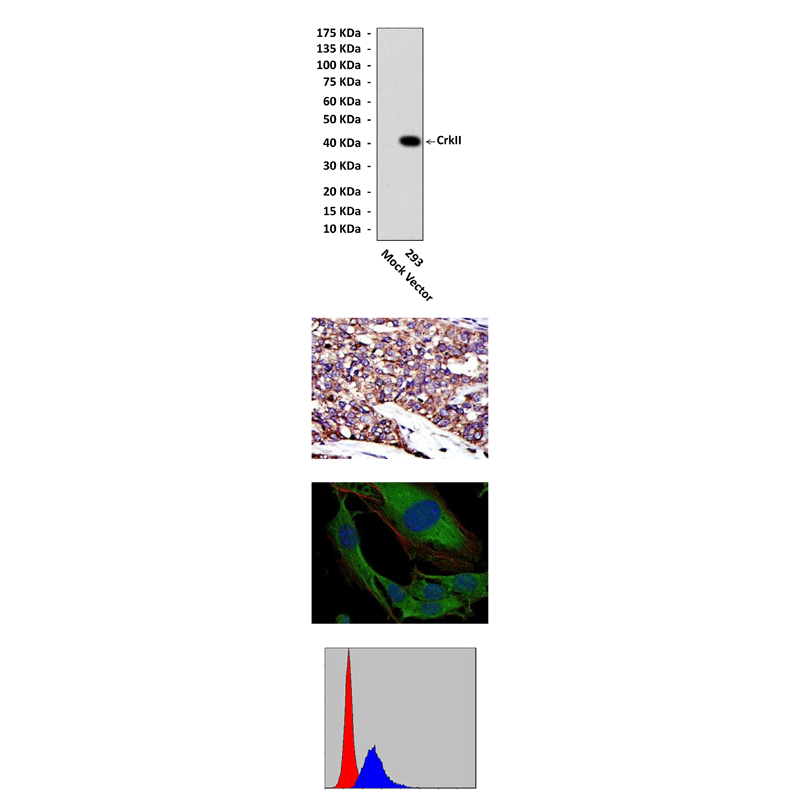
Anti-CrkII: Mouse CrkII Antibody |
 |
BACKGROUND Crk was originally isolated as the oncogene fusion product of the CT10 chicken retrovirus (v-Crk). Cellular homologues of v-Crk include the c-CRK gene, which encodes two alternatively spliced mRNAs that give rise to two proteins (c-CrkI and c-CrkII), and a second gene, c-CRKL. Crk proteins are composed of one Src homology 2 (SH2) and, one or two Src homology 3 (SH3) domains (CrkI, lacking the SH3-C due to the alternate splicing of CrkII mRNA) and lack intrinsic enzymatic activity. They are adaptor proteins that direct the assembly of multiprotein signaling complexes. Crk adaptor proteins and their effectors are highly conserved throughout evolution. The Crk SH2 domain binds a specific phosphorylated tyrosine motif found in proteins involved in cell spreading, actin reorganization, and cell migration. These Crk-SH2 binding proteins include the focal adhesion components, p130Cas and paxillin, growth factor receptor tyrosine kinases, and a docking protein Gab1, involved in epithelial dispersal and morphogenesis. The NH2-terminal SH3 domain of CrkII interacts constitutively with proline-rich motifs present within several proteins, including C3G, a nucleotide exchange factor for Rap1, DOCK180, an exchange factor for Rac1, as well as the Abl tyrosine kinase, tyrosine phosphatase (protein tyrosine phosphatase 1B), the p85 subunit of phosphatidylinositol 3-kinase , and JNK. The inducible binding of the Crk SH2 domains to phosphotyrosine motifs contained in the target proteins is believed to localize effector proteins bound constitutively to the Crk SH3 domains.1 It was demonstrated that CRKI/II adaptor proteins are critical integrators of upstream signals for cell invasion and migration in human cancer cell lines.2
CrkII is tyrosylphosphorylated following the activation of the nerve growth factor receptor (trkA), the B-cell antigen receptor (BCR), and the IGF-IR. Two nonreceptor kinases, c-Arg and c-Abl, have been shown to tyrosine phosphorylate Crk. The tyrosylphosphorylation of tyrosine 221 or 222, depending on the species, located between the SH3-N and SH3-C of CrkII results in the formation of an intramolecular binding site for the SH2 domain. The formation of this intramolecular bond is believed to prevent the N-terminal SH2 and SH3 domains from associating with other proteins. Thus tyrosine phosphorylation represents a possible mechanism to control the protein binding activity of CrkII.3 Since a similar tyrosine-containing motif is also present in CrkL, the phosphorylation of this tyrosine (tyrosine 207) may regulate CrkL binding activity by a similar mechanism.4
CrkII is tyrosylphosphorylated following the activation of the nerve growth factor receptor (trkA), the B-cell antigen receptor (BCR), and the IGF-IR. Two nonreceptor kinases, c-Arg and c-Abl, have been shown to tyrosine phosphorylate Crk. The tyrosylphosphorylation of tyrosine 221 or 222, depending on the species, located between the SH3-N and SH3-C of CrkII results in the formation of an intramolecular binding site for the SH2 domain. The formation of this intramolecular bond is believed to prevent the N-terminal SH2 and SH3 domains from associating with other proteins. Thus tyrosine phosphorylation represents a possible mechanism to control the protein binding activity of CrkII.3 Since a similar tyrosine-containing motif is also present in CrkL, the phosphorylation of this tyrosine (tyrosine 207) may regulate CrkL binding activity by a similar mechanism.4
REFERENCES
1. Butler, A.A. et al: J. Biol. Chem. 272:27660-64, 1997
2. Rodrigues, S.P. et al: Mol. Cancer Res. 3:193-94, 2005
3. Feller, S.M. et al: EMBO J. 13:2341-51,1994
4. Wange, R.L. : Curr. Biol. 14:R562-4, 2004
2. Rodrigues, S.P. et al: Mol. Cancer Res. 3:193-94, 2005
3. Feller, S.M. et al: EMBO J. 13:2341-51,1994
4. Wange, R.L. : Curr. Biol. 14:R562-4, 2004
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10403 |
Antigen: | Raised against recombinant human CrkII fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC 1:50 - 1:200 FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 40 kDa |
Specificity/Sensitivity: | Detects CrkII proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой