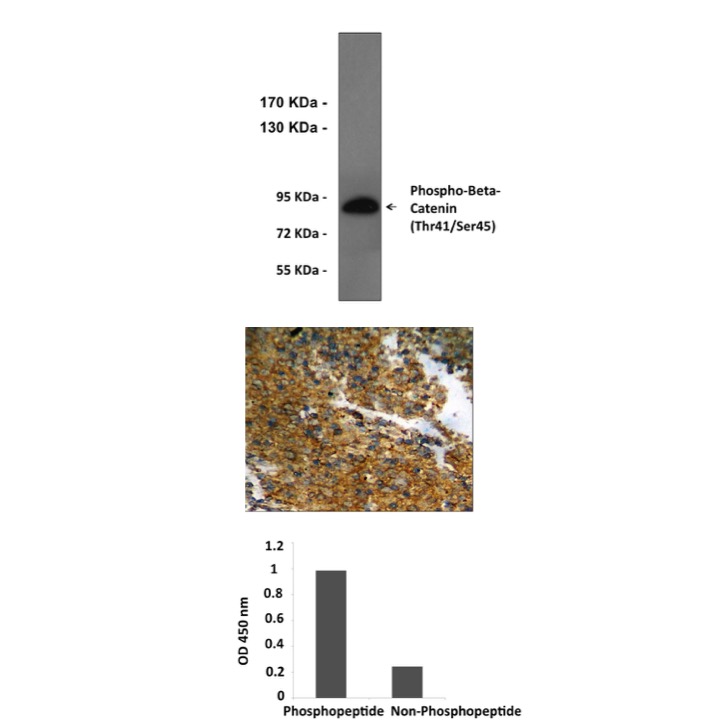
Anti-Phospho-β-Catenin: Rabbit Beta-Catenin, Phospho-Thr41/Ser45, Antibody |
 |
BACKGROUND The protein beta-Catenin was first described in humans as a member of the cell membrane-bound adherens complex. A second role for beta-Catenin in cell-signaling was discovered, which involves translocation of this protein from the cytoplasm into the nucleus. beta-Catenin may be regarded as existing in three different subcellular forms: membrane-bound (as part of the adherens complex), cytosolic, and nuclear. Binding of the protein to other members of the adherens complex, ie, E-cadherin and alpha-Catenin, is thought to be regulated by tyrosine phosphorylation. Tyrosine phosphorylation of beta-Catenin leads to its dissociation from the adherens complex to the cytosol.1 Cytosolic beta-Catenin may subsequently be translocated into the nucleus or be degraded. In nucleus, beta-Catenin binds with a member of the TCF/LEF family of transcription factors to form a complex that activates transcription of target genes by binding to their promoter sequences.2 The degradation of beta-Catenin involves binding of the protein to a complex involving APC protein, and two further proteins, AXIN and glycogen synthase kinase (GSK)-3beta. The latter serves to phosphorylate serine and threonine residues on beta-Catenin (Thr41, Ser37 and Ser33), a crucial step required to target the protein for ubiquitination and proteosomal degradation. Both APC and AXIN enhance this phosphorylation. Moreover, CK1 phosphorylates Ser45 of beta-Catenin generating a priming site for the subsequent phosphorylation by GSK-3beta. Phosphorylation of beta-Catenin is important in enabling binding to the F box protein beta-TrCP and hence ubiquitin-mediated proteolysis.3 Wnt signaling pathway plays important role in regulation of this process.4 Binding of Wnt family glycoproteins to their trans-membrane receptor, Frz, leads to increased activity of the protein Dishevelled (Dvl) that, in turn, inhibits GSK-3beta phosphorylating activity, which leads to increase of cytosolic beta-Catenin and its nuclear translocation. However, it has recently been shown that beta-Catenin may also be targeted for such degradation independent of GSK-3beta-mediated phosphorylation. This putative alternative pathway requires interaction between beta-Catenin, APC, and a complex of proteins including the p53-inducible protein, Siah-1.5
REFERENCES
1. Hinck, L. et al: Trends in Biochem Sci. 19:538-542, 1994
2. Alexander, N. et al: Am. J. Path. 160:389-401, 2002
3. Mulholland, D.J. et al:Endocrin. Rev. 26:898-915, 2005
4. Clevers, H.: Cell 127:469-480, 2006
5. Liu, J. et al: Mol. Cell 7:927-36, 2001
2. Alexander, N. et al: Am. J. Path. 160:389-401, 2002
3. Mulholland, D.J. et al:Endocrin. Rev. 26:898-915, 2005
4. Clevers, H.: Cell 127:469-480, 2006
5. Liu, J. et al: Mol. Cell 7:927-36, 2001
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1042 |
Antigen: | Range AA30 to 60 |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:500-1:1000 IP n/d IHC 1:50-1:100 ICC n/d FACS n/d ELISA 1:20000 |
Predicted Molecular Weight of protein: | 85 KDa |
Specificity/Sensitivity: | Detects endogenous levels of Beta-Catenin without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой