Anti-EphA7: Mouse EphA7 Antibody |
 |
BACKGROUND Eph family of receptor tyrosine kinases consists of at least 14 distinct receptors and has eight membrane-bound ligands, known as the ephrins. This is the largest family of receptor tyrosine kinases. Eph proteins are divided into two subfamilies: the EphA receptors (A1-A8) that bind glycosyl phosphatidylinositol (GPI)-linked ephrin-A ligands (A1-A5), and the EphB receptors (B1-B6) that bind transmembrane ephrin-B ligands (B1-B3). The only known crosstalk between the A and B subfamilies occurs with the EphA4 receptor, which can bind ephrins-B2 and -B3 as well as the entire A subclass. There is a great deal of redundancy of receptor-ligand binding specificity within each subfamily, although binding affinities vary.1,2 Both GPI-anchoredephrinA and transmembrane ephrinB ligands interact with the Nterminal globular domain (Glob) of Eph receptors. The Eph receptors become phosphorylated at specific tyrosine residues in the cytoplasmic domain following ligand binding. Phosphorylated motifs serve as sites of interaction with certain cytoplasmic signaling proteins to mediate downstream signaling. In addition, through their C terminus the Eph receptors associate with PDZ (postsynaptic density protein, disc large, zona occludens) domain-containing proteins. Moreover, Eph receptor contact induces tyrosine phosphorylation of the cytoplasmic domain of ephrinB proteins via an SRC-family kinase (SFK), which mediating the reverse signaling. One of the unique features of Eph/ephrin signaling is the fact that both receptors and ligands are competent to transduce a signaling cascade upon interaction. Eph-activated signaling is termed forward, and ephrin-activated signaling is termed reverse. Another level of complexity stems from the fact that interactions between Eph receptors and ephrins can happen in trans (between two opposing cells) or in cis (within the same cell). It is commonly assumed that trans interactions are activating while cis interactions are inhibiting.3 Eph-Ephrin signaling functions in a variety biological processes including diverse assegmentation of the somites and rhombomeres, the formation of blood vessels, Axon guidance and fasciculation, migration of the neural crest and metastasis of transformed cells etc.
EphA7, previously known as Mdk1, Hek11, Ehk-3, Ebk, and Cek11, was originally isolated from adult mouse brain. In mice, EphA7 is expressed not only in its conventional full-length version, containing the intracellular tyrosine kinase domain but also in a truncated form that lacks this domain. EphA7 is expressed early in cortical development, becoming concentrated in anterior and posterior domains. The full-length and truncated isoforms of Eph7 are repulsive and adhesive, respectively. EphA7 protein may take part in the regulation of cell differentiation in colorectal cancer and the downregulation of EphA7 is an early event during the initiation of colorectal carcinogenesis.4
REFERENCES
1. Brantley-Sieders, D.M. & Chen, J.: Angiogenesis. 7:17, 2004
2. Murai, K.K. & Pasquale, E.B.: J. Cell Sci. 116:2823, 2003
3. Arvanitis, D. & Davy, A.: Genes & Dev. 22:416, 2008
4. Wang, J. et al: Oncogene 24:5637-47, 2005
2. Murai, K.K. & Pasquale, E.B.: J. Cell Sci. 116:2823, 2003
3. Arvanitis, D. & Davy, A.: Genes & Dev. 22:416, 2008
4. Wang, J. et al: Oncogene 24:5637-47, 2005
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10082 |
Antigen: | Purified recombinant human EphA7 fragments expressed in E. coli. |
Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 128 kDa |
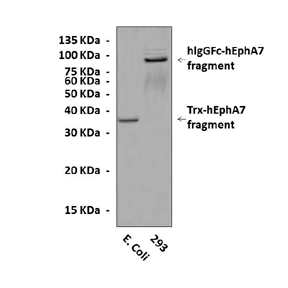
Specificity/Sensitivity: | Detects overexpressed EphA7 proteins in cells without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой