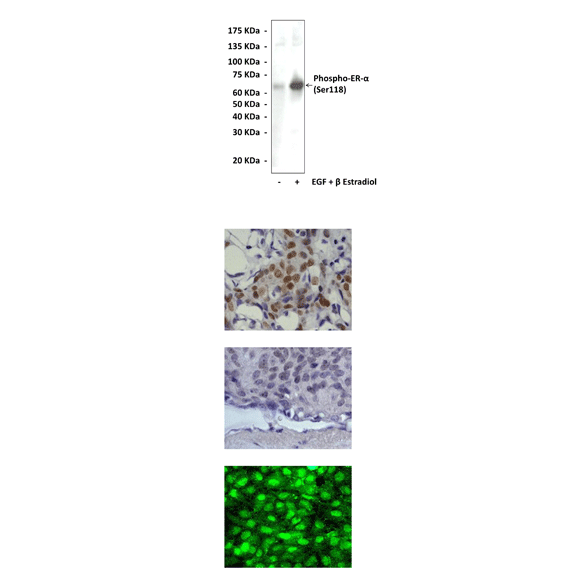
Anti-Phospho-ER-α: Rabbit ER-alpha, Phospho-Ser118 Antibody |
 |
BACKGROUND Estrogen receptors (ERs), of which there are two major subtypes, alpha and beta, are multidomain transcription regulators whose activity is regulated by the binding of ligands. Both receptor subtypes have overlapping but also unique roles in estrogen-dependent action in vivo. Additionally, ERalpha and ERbeta have different transcriptional activities in certain ligand, cell-type, and promoter contexts. When coexpressed, ERbeta exhibits an inhibitory action on ERalpha. -mediated gene expression and in many instances opposes the actions of ERalpha. A number of ERalpha and ERbeta isoforms have also been described, many of which alter estrogen-mediated gene expression. ER-alpha has three splicing isoforms: full-length ER-alpha66, ER-alpha46, and ER-alpha36. The shorter 46-kDa isoform of hER lacks exon 1 and consequently the N-terminal AF-1 region. This isoform heterodimerizes with wild-type ER-alpha66, thereby suppressing its AF-1–dependent transcriptional activity. ER-alpha36 predominantly localizes on the plasma membrane and in cytoplasma, and lacks intrinsic transcription activity and mainly mediates nongenomic estrogen signaling.1
ER-mediated gene transcription is regulated not only by the binding of estrogens or SERMs to the ER but also by other post-translational events, such as receptor phosphorylation, which can be induced both by the binding of estrogen and SERMs and by mitogen-activated protein kinase (MAPK) pathways. ER-alpha is phosphorylated on multiple amino acid residues. Ser104, 106, 118, and 167 are all located within the activation function (AF)1 region of ER-alpha, and their phosphorylation provides the important mechanism that regulates AF1 activity.2 ER function is also modulated by interactions with coactivators and corepressors that can either positively or negatively modulate ERalpha-mediated transcriptional activity. Growth factor signaling (e.g. EGFR/Her2) may stimulate ER signaling via activate both ER and the important ER coactivator AIB1/SRC-3.3
It is prevailingly considered that ER acts as a transcription factor that is mainly localized in the cell nucleus. However, accumulating evidence has demonstrated that ER also exists on the plasma membrane and participates in rapid estrogen signaling. Association of ER and caveolin-1 also was shown to facilitate ER localization on the plasma membrane in caveolae. It was postulated that estrogen may rapidly activate different signaling pathways, including MAPK/ERK, phospholipase C, PI3K/Akt and G protein-coupled receptor-activated pathways in the caveolae.4
REFERENCES
1. Lin, S.L. et al: PLoS ONE 5:e9103, 2010
2. Yamashita, H. et al: Breast Cancer Res.7:R753-R764, 2005
3. Osborne, C.K. & Schiff, R.: Breast 12:362-7, 2003
4. Sotgia, F. et al: Cancer Res.66:10647-51, 2006
2. Yamashita, H. et al: Breast Cancer Res.7:R753-R764, 2005
3. Osborne, C.K. & Schiff, R.: Breast 12:362-7, 2003
4. Sotgia, F. et al: Cancer Res.66:10647-51, 2006
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1144 |
Antigen: | Short peptide from human ER-alpha sequence surrounding and containing phospho-Ser118. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 ICC 1:50 - 1:100 FACS n/d |
Predicted Molecular Weight of protein: | 66 kDa |
Specificity/Sensitivity: | Detects phosphorylated ER-alpha (Ser118) proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой