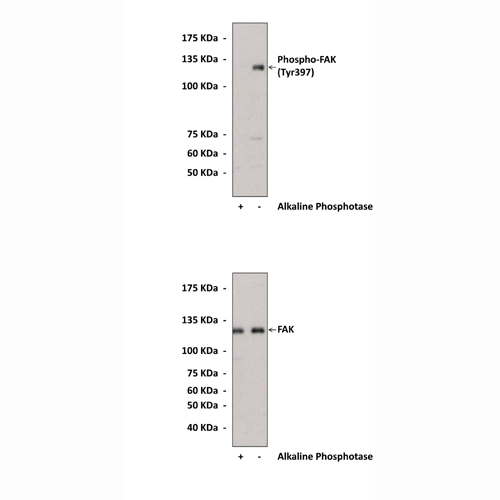
Anti-Phospho-FAK: Polyclonal Focal Adhesion Kinase, Phospho-Tyr397 Antibody |
 |
BACKGROUND Focal adhesion kinase (FAK) is a protein tyrosine kinase which is recruited at an early stage to focal adhesions and which mediates many of the downstream responses. Integrin stimulation induces FAK autophosphorylation at Tyr397, creating a binding site for the src homology 2 (SH2) domain of the Src-PTKs src or fyn. The recruited, active Src-PTK phosphorylates several other tyrosine residues in FAK to affect both full FAK activation and the creation of phosphotyrosine binding sites for other signaling molecules, including the adaptor protein Grb2 and the p85-subunit of phosphatidylinositol 3 kinase (PI3 kinase).1,2 The Src–PTK–FAK complex mediates the phosphorylation of certain FAK-associated proteins such as p130CAS and paxillin, and their recruitment of other signaling molecules (such as phosphotyrosine-dependent Crk binding to p130CAS and paxillin, and Nck binding to p130CAS), leading to formation of a multi-phosphocomponent signaling complex localized at focal adhesions.3 Given that the development of malignancy is often associated with perturbations in these processes, it is not surprising that FAK activity is altered in cancer cells. Mouse models have shown that FAK is involved in tumor formation and progression, and other studies showing that FAK expression is increased in human tumors make FAK a potentially important new therapeutic target.
REFERENCES
1. Schaller MD et al.: Proc Natl Acad Sci U S A. 89:5192, 1992.
2. Calalb MB et al.: Mol Cell Biol. 15:954, 1995.
3. Law SF et al.: Mol Cell Biol. 16:3327, 1996.
2. Calalb MB et al.: Mol Cell Biol. 15:954, 1995.
3. Law SF et al.: Mol Cell Biol. 16:3327, 1996.
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CB3874 |
Antigen: | KLH-conjugated peptide containing human FAK Tyr397 surrounding sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC (paraffin) n/d ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 125 kDa |
Specificity/Sensitivity: | Detects endogenous levels of FAK proteins when phosphorylated at Tyr397. Does not cross-react with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой