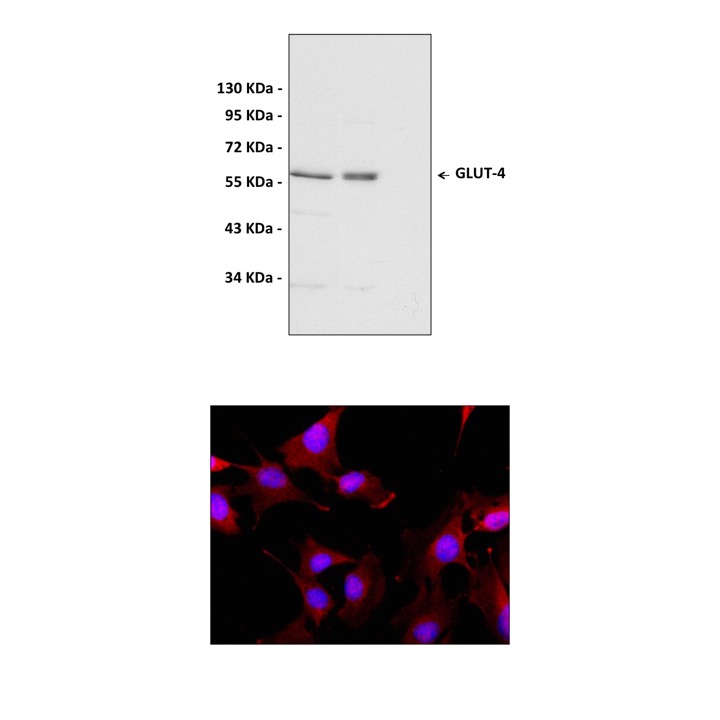
Anti-GLUT-4: Rabbit GLUT-4 Antibody |
 |
BACKGROUND Glucose transport in mammalian cells is mediated by a family of structurally related glycoproteins, the glucose transporters (GLUTs). Based on the sequence and functional similarities, GLUT family can be divided into three subfamilies, namely class I (the previously known glucose transporters GLUT1-4), class II (the previously known fructose transporter GLUT5, the GLUT7, GLUT9 and GLUT11), and class III (GLUT6, 8, 10, 12, and the myo-inositol transporter HMIT1).1
GLUT4 is the major insulin-responsive transporter that is predominantly restricted to striated muscle and adipose tissue. In contrast to the other GLUT isoforms, which are primarily localized to the cell surface membrane, GLUT4 transporter proteins are sequestered into specialized storage vesicles that remain within the cell's interior under basal conditions. As postprandial glucose levels rise, the subsequent increase in circulating insulin activates intracellular signaling cascades that ultimately result in the translocation of the GLUT4 storage compartments to the plasma membrane. Importantly, this process is readily reversible such that when circulating insulin levels decline, GLUT4 transporters are removed from the plasma membrane by endocytosis and are recycled back to their intracellular storage compartments. Therefore, by establishing an internal membrane compartment as the default localization for the GLUT4 transporters, insulin-responsive tissues are poised to respond rapidly and efficiently to fluctuations in circulating insulin levels. Unfortunately, the complexity of these regulatory processes provides numerous potential targets that may be defective and eventually result in peripheral tissue insulin resistance and possibly diabetes.2 As such, understanding the molecular details of GLUT4 expression, GLUT4 vesicle compartment biogenesis, GLUT4 sequestration, vesicle trafficking, and fusion with the plasma membrane has become a major focus for many laboratories. Gaps remain in our understanding of the precise molecular mechanisms by which insulin regulates glucose uptake in fat and muscle cells. Recent evidence suggests that insulin action involves multiple pathways, each compartmentalized in discrete domains. Upon activation, the receptor catalyzes the tyrosine phosphorylation of a number of substrates. One family of these, the insulin receptor substrate (IRS) proteins, initiates activation of the phosphatidylinositol 3-kinase pathway, resulting in stimulation of protein kinases such as Akt and atypical protein kinase C. The receptor also phosphorylates the adapter protein APS, resulting in the activation of the G protein TC10, which resides in lipid rafts. TC10 can influence a number of cellular processes, including changes in the actin cytoskeleton, recruitment of effectors such as the adapter protein CIP4, and assembly of the exocyst complex. These pathways converge to control the recycling of the facilitative glucose transporter Glut4.3
REFERENCES
1. Joost, H.G. & Thorens, B.: Mol Membr Biol. 18:247-56, 2001
2. Huang, S. & Czech, M.: Cell Metabol. 5:237-52, 2007
3. Chang, L. et al: Mol. Med. 10:65-71, 2004
2. Huang, S. & Czech, M.: Cell Metabol. 5:237-52, 2007
3. Chang, L. et al: Mol. Med. 10:65-71, 2004
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1182 |
Antigen: | Synthetic peptide corresponding to amino acids 493-509 of human GLUT4, conjugated to KLH. The corresponding sequence is identical in rat and mouse GLUT4. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse |
Applications & Suggested starting dilutions:* | WB 1-2 ug/mL IP n/d IHC n/d ICC n/d FACS n/d IF 15-20 ug/mL |
Predicted Molecular Weight of protein: | 58 kDa |
Specificity/Sensitivity: | Detects endogenous GLUT-4 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой