Anti-GST: Mouse Glutathione S-Transferase Antibody |
 |
BACKGROUND GST is commonly used to create fusion proteins.1 The tag has the size of 220 amino acids (roughly 26 KDa), which, compared to other tags like the myc- or the FLAG-tag, is quite big. It is fused to the N-terminus of a protein. However, many commercially-available sources of GST-tagged plasmids include a thrombin domain for cleavage of the GST tag during protein purification.
A GST-tag is often used to separate and purify as well as confirm expression of proteins that contain the GST-fusion. GST-fusion proteins can be produced in Escherichia coli, as recombinant proteins. The GST part binds its substrate, glutathione. Agarose beads can be coated with glutathione, and such glutathione-Agarose beads bind GST-proteins. These beads are then washed, to remove contaminating bacterial proteins. Adding free glutathione to beads that bind purified GST-proteins will release the GST-protein in solution. GST-tag antibody is a useful tool for confirming protein expression, localization of expressed proteins in cells, as well as affinity-binding of GST-tagged proteins.2
REFERENCES
1. Scheich, C. et al: BMC Biotechnol. 3:12, 2003
2. Nojima, H. et al: J. Biol. Chem. 278:15461-4, 2003
2. Nojima, H. et al: J. Biol. Chem. 278:15461-4, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10120 |
Antigen: | Purified recombinant GST-tag expressed in E. coli. |
Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | GST-Tag |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC n/d ICC n/d FACS n/d |
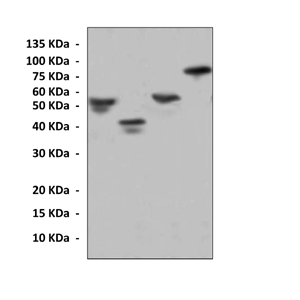
Predicted Molecular Weight of protein: | 26 kDa (GST-tag) |
Specificity/Sensitivity: | Detects endogenous proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой