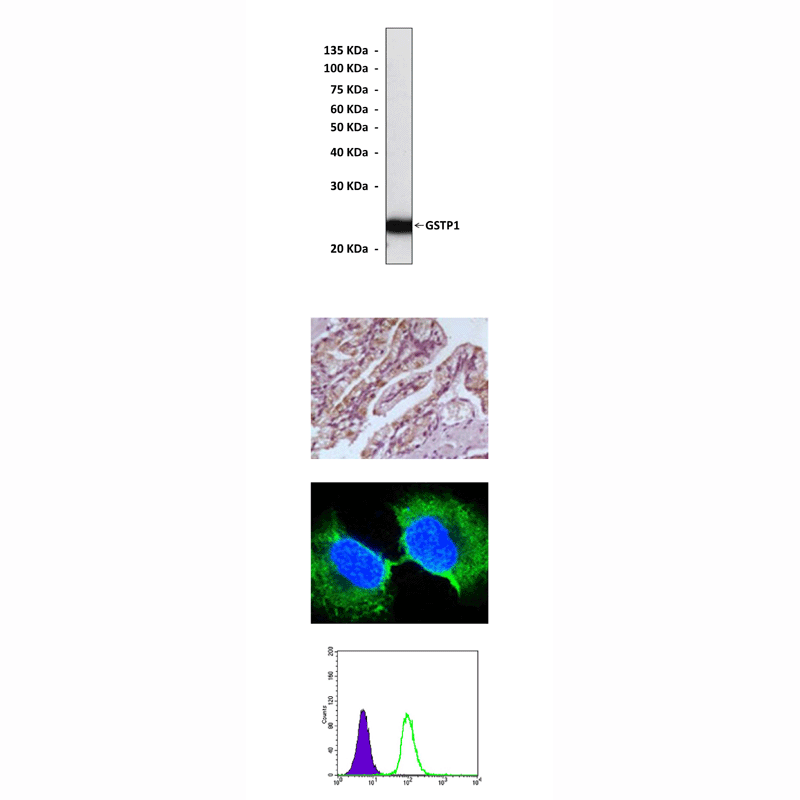
Anti-GSTP1: Mouse Glutathione S-Transferase Pi 1 Antibody |
 |
BACKGROUND Glutathione S-transferases (GSTs) are a large and diverse family of enzymes, and in humans, there are at least 13 GST enzymes belonging to five families, namely alpha (GSTA), mu (GSTM), pi (GSTP), theta (GSTS), and tao (GSTT). GSTs play an important role in detoxification by catalyzing the conjugation of many hydrophobic and electrophilic compounds with reduced glutathione.
Among this family of isozymes, GSTP1 is generally the most prevalent in mammalian cells. The link between GST overexpression, especially with respect to GSTP1, and anticancer drug resistance has been extensively studied. Because of the defined role of GST in drug metabolism, elevated expression of GSTP1 in solid tumors or in drug-resistant cells has been associated frequently with detoxification reactions even in instances where there is no evidence that the selecting drug is a substrate for GSTP1. More recently, however, the link between the redox active components of GSTP1 and stress-activated kinases, such as JNK, has been redefined as a non-catalytic, ligand binding activity that mediates both stress and apoptotic responses. In a parallel series of studies, either GSTmu or thioredoxin has also been identified as a ligand-binding partner for apoptosis-signaling kinase (ASK1), extending the role that “redox proteins” may have in kinase regulation. It was demonstrate that GSTP1 has significant affinity for the C terminus of JNK and confirms the ligand-binding regulatory role for this ubiquitously expressed protein.1 In addition; GSTP1 activity can be regulated by interacting with other proteins. The Fanconi anemia group C protein (FANCC) interacts with GSTP1 and prevents the formation of inactivating disulfide bonds within GSTP1 and increases GSTP1 activity during apoptosis.2
GSTP1 is not only involved in cancers, but also in other diseases. GSTP1 gene polymorphisms affect substrate selectivity and stability, and the oxidative milieu in dopaminergic neurons, which increases the susceptibility to Parkinson’s disease. In addition, it is possible that intermediate electrophilic metabolites, arising in the first phase of detoxification, are not metabolized by GST enzymes in asthmatic patients and are not excreted. These intermediate metabolites may damage cells and generate oxidative stress, and so contribute to the pathogenesis of asthma.3
REFERENCES
1. Wang, T. et al: J. Biol. Chem. 276:20999-21003, 2001
2. Cummings, R.C. et al: Nature Med. 7:814-20, 2001
3. Tamer, L. et al: Respiration 9:493-8, 2004
2. Cummings, R.C. et al: Nature Med. 7:814-20, 2001
3. Tamer, L. et al: Respiration 9:493-8, 2004
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10121 |
Antigen: | Purified recombinant human GSTP1 proteins expressed in E. coli. |
Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:200 ICC 1:200 FACS 1:200 |
Predicted Molecular Weight of protein: | 23 kDa |
Specificity/Sensitivity: | Detects endogenous GSTP1 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой