Anti-Hsp40: Rabbit Heat Shock Protein 40 kDa Antibody |
 |
BACKGROUND Hsp40 (Heat Shock Protein 40 kDa, also known as DnaJ) family of heat shock proteins are expressed in a wide variety of organisms from bacteria to humans. Hsp40/DnaJ proteins are important for protein translation, folding, unfolding, translocation, and degradation, primarily by stimulating the ATPase activity of chaperone proteins, Hsp70s. Because the ATP hydrolysis is essential for the activity of Hsp70s, Hsp40/DnaJ proteins actually determine the activity of Hsp70s by stabilizing their interaction with substrate proteins. Members of the Hsp40/DnaJ family of proteins have three distinct domains, (i) a highly conserved J domain of approximately 70 amino acids, often near the N terminus, that is responsible for the interaction with Hsp70, (ii) a glycine and phenylalanine (G/F)-rich region that putatively acts as a flexible linker; and (iii) a cysteine-rich, zinc finger-containing C-terminal domain. Two distinct sets of cofactors tightly monitor Hsp70 action by regulating ATPase activity: J domain-containing proteins (JDPs) of the Hsp40/DnaJ family and nucleotide exchange factors. The highly conserved J domain of Hsp40/DnaJ proteins contacts the nucleotide-binding domain of Hsp70 and enhances ATPase activity by inducing a conformational change. This leads to enhanced binding of Hsp70s to substrates. Moreover, Hsp40/DnaJ proteins directly bind to unfolded regions on substrate proteins through their substrate-binding domain and deliver the unfolded protein to the ATP-bound form of their Hsp70 partner, whereas others contain atypical domains that specify exclusive functions. The nucleotide exchange factors, on the other hand, release bound ADP, which triggers ATP rebinding and subsequent substrate release from the Hsp70.1 In contrast, negative regulators compete with Hsp40/DnaJ co-chaperones and consequently convert hsp70 from a protein-refolding machine into a factor that targets the substrate protein to the ubiquitin-proteasome system (UPS). Bcl-2-associated athanogene-1 (Bag-1) proteins and the E3 ligase carboxyl-terminus of hsp70-interacting protein (CHIP) are examples of negative regulators, and they form an important link between the chaperone and ubiquitin-protein degradation systems.2
Hsp40 is a member of Hsp40/DnaJ protein family. Hsp40/DnaJ proteins can be categorized into three groups, depending on the presence of other domains. Genome-wide analysis has revealed 41 DnaJ/Hsp40 family members (or putative members) in humans. While 34 contain the typical J domains, 7 bear partially conserved J-like domains, but are still suggested to function as DnaJ/ Hsp40 proteins.3
Hsp40 is a member of Hsp40/DnaJ protein family. Hsp40/DnaJ proteins can be categorized into three groups, depending on the presence of other domains. Genome-wide analysis has revealed 41 DnaJ/Hsp40 family members (or putative members) in humans. While 34 contain the typical J domains, 7 bear partially conserved J-like domains, but are still suggested to function as DnaJ/ Hsp40 proteins.3
REFERENCES
1. Vembar, S.S. et al: J. Biol. Chem. 284:32462-71, 2009
2. Howarth, J.L. et al: Mol. Therapy 15:1100-5, 2007
3. Xiu, X.B. et al: Mol. Cell. Life Sci. 63:2560-70, 2006
2. Howarth, J.L. et al: Mol. Therapy 15:1100-5, 2007
3. Xiu, X.B. et al: Mol. Cell. Life Sci. 63:2560-70, 2006
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CA1043 |
Antigen: | Short peptide from human Hsp40 sequence. |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS n/d |
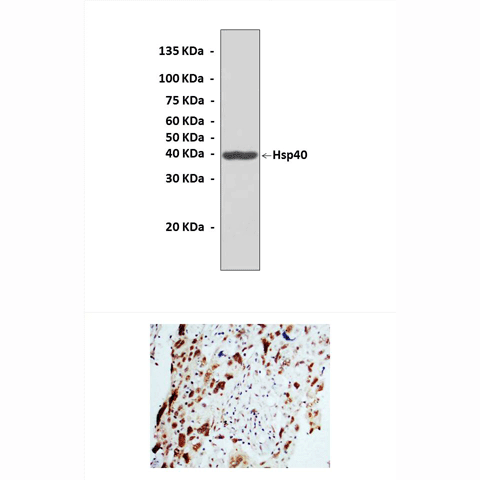
Predicted Molecular Weight of protein: | 40 kDa |
Specificity/Sensitivity: | Detects endogenous levels of Hsp40 proteins without cross-reactivity with other related proteins. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой