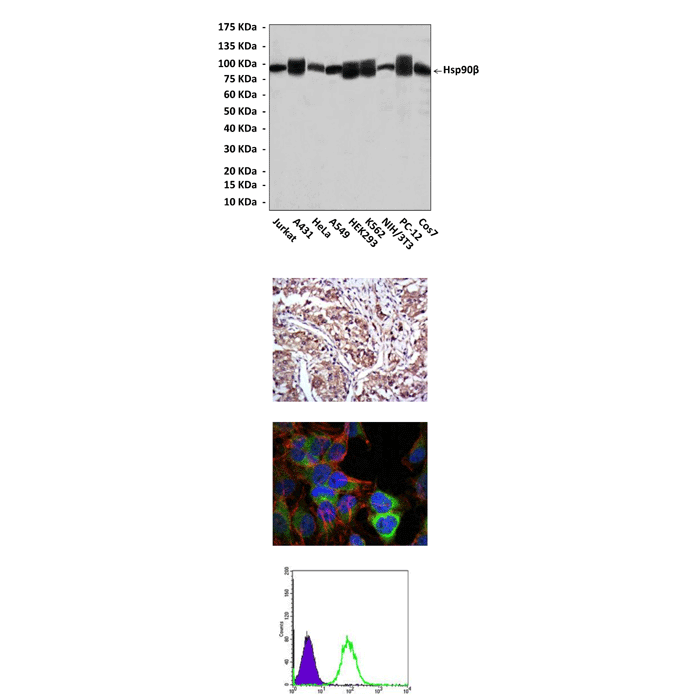
Anti-Hsp90-β: Mouse Hsp90-beta Antibody |
 |
BACKGROUND The highly conserved Hsp90 chaperone family includes the eponymous Hsp90 (90 kDa heat shock protein) of the eukaryotic cytosol, termed variously Hsp90alpha and beta in humans (corresponding to a major and minor isoform), Hsp86 and Hsp84 in mice. Hsp90 plays key roles in signal transduction, protein folding, protein degradation, and morphologic evolution.1
Functional Hsp90 operates as dimer and has intrinsic ATPase activity. At a molecular level, Hsp90 binds to substrate proteins, which are in a near native state and thus at a late stage of folding poised for activation by ligand binding or interaction with other factors. In fulfilling its role, Hsp90 operates as part of a multichaperone machinery in the cytosol, which includes other chaperones (e.g. Hsp70) and peptidyl-prolyl isomerases, and is regulated by a number of co-chaperones/accessory proteins (e.g. Hop, cdc37).2 Hsp90 has been shown to aid protein folding and quality control for a large number of client proteins. Unlike Hsp70, eukaryotic cytosolic Hsp90 does not act generally in nascent protein Hsp90 is distinguished from other chaperones in that most of its known substrates are signal transduction proteins.3 It interacts with >100 proteins and some notable clients include kinases (e.g. Raf-1), nuclear hormone receptors (e.g. estrogen receptors), transcription factors (e.g. p53), GPCRs (e.g. CB2 receptors) and ion channels (e.g. CFTR). In humans, the Hsp90beta isoform is constitutively expressed whereas the Hsp90alpha isoforms is expressed under stress conditions. Hsp90 plays an important role in some tumor cell types by stabilizing mutated oncogenic proteins.4
Functional Hsp90 operates as dimer and has intrinsic ATPase activity. At a molecular level, Hsp90 binds to substrate proteins, which are in a near native state and thus at a late stage of folding poised for activation by ligand binding or interaction with other factors. In fulfilling its role, Hsp90 operates as part of a multichaperone machinery in the cytosol, which includes other chaperones (e.g. Hsp70) and peptidyl-prolyl isomerases, and is regulated by a number of co-chaperones/accessory proteins (e.g. Hop, cdc37).2 Hsp90 has been shown to aid protein folding and quality control for a large number of client proteins. Unlike Hsp70, eukaryotic cytosolic Hsp90 does not act generally in nascent protein Hsp90 is distinguished from other chaperones in that most of its known substrates are signal transduction proteins.3 It interacts with >100 proteins and some notable clients include kinases (e.g. Raf-1), nuclear hormone receptors (e.g. estrogen receptors), transcription factors (e.g. p53), GPCRs (e.g. CB2 receptors) and ion channels (e.g. CFTR). In humans, the Hsp90beta isoform is constitutively expressed whereas the Hsp90alpha isoforms is expressed under stress conditions. Hsp90 plays an important role in some tumor cell types by stabilizing mutated oncogenic proteins.4
REFERENCES
1. Bose, S. et al: Science 274:1715-7, 1996
2. Young, J.C. et al: J. Cell Sci. 154:267-74, 2001
3. Picard, D. et al: Nature 348:166-8, 1990
4. Sidera, K. et al: J. Biol. Chem. 283:2031-41, 2008
2. Young, J.C. et al: J. Cell Sci. 154:267-74, 2001
3. Picard, D. et al: Nature 348:166-8, 1990
4. Sidera, K. et al: J. Biol. Chem. 283:2031-41, 2008
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
|
Cat.No.:
|
CP10397
|
|
Antigen:
|
Recombinant human Hsp90-beta fragments expressed in E. coli.
|
|
Isotype:
|
Mouse IgG1
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human, Mouse, Rat
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:1000
IP 1:50
IHC 1:50 - 1:200
ICC 1:50 - 1:200
FACS 1:50 - 1:200
|
|
Predicted Molecular
Weight of protein:
|
90 kDa
|
|
Specificity/Sensitivity:
|
Detects Hsp90-beta proteins in various cell lysate.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой