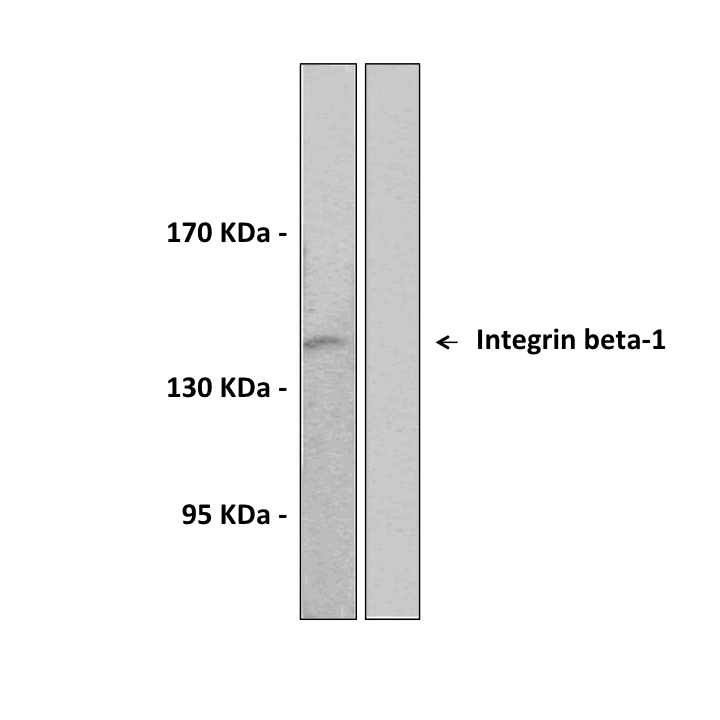
Anti-Integrin-β1: Rabbit Integrin beta-1 Antibody |
 |
BACKGROUND The integrins are a family of alpha/betab heterodimeric receptors that mediate dynamic linkages between extracellular adhesion molecules and the intracellular actin cytoskeleton. Integrins are expressed by all multicellular animals, but their diversity varies widely among species; for example, in mammals, 19 alpha and 8 beta subunit genes encode polypeptides that combine to form 25 different receptors. Both integrin subunits are type I transmembrane proteins with large extracellular and short cytoplasmic domains of 700-1100 and 30-50 residuesrespectively. Integrins are ubiquitously expressed and at physiological conditions, integrins are highly glycosylated and contain a Ca2+ or Mg2+ ion, which is essential for ligand binding. Integrin receptors are critical for cell attachment to the extracellular matrix (ECM) and this is mediated through integrin-fibronectin, -vitronectin, -collagen and -laminin interactions. Intracellularly, integrins form adhesion complexes with proteins including talin, vinculin, paxillin and alpha-actinin. They also regulate kinases, such as focal adhesion kinase and Src family kinases, to mediate attachment to the actin cytoskeleton. Integrins also have a significant role in cell signaling and can activate protein kinases involved in the regulation of cell growth, division, survival, differentiation, migration and apoptosis. The beta 1, beta 3, and beta 5 integrin intracellular domains are sufficient to initiate signal transduction pathways. Furthermore, alternative splicing can regulate the ability of beta integrin intracellular domains to participate in signal transduction. Glycoprotein II/IIIb (alphaIIbbeta3) is an integrin receptor found on the surface of platelets. It is involved in the cross-linking of platelets with fibrin, and so has a vital role in blood clot formation.1
The integrin beta-1 (CD29) molecules are associated with one of at least 12 different alpha subunits. integrin beta-1 molecules can form receptors for fibronectin, collagen, and laminin, VCAM1, cytotactin, osteopontin, epiligrin, thrombospondin, CSPG4, vitronectin and also the human T cell very late antigen (VLA) 1 heterodimers. The high-affinity fibronectin receptor is alpha5/beta1 (or VLA-5).2
Integrin beta-1 recognize the sequence R-G-D in a wide array of ligands. Isoform beta-1B interferes with isoform beta-1A resulting in a dominant negative effect on cell adhesion and migration. It is involved in promoting endothelial cell motility and angiogenesis. It also play important role in up-regulation of the activity of kinases such as PKC via binding to KRT1. Together with KRT1 and GNB2L1/RACK1, it serves as a platform for Src activation or inactivation. In addition, it plays a mechanistic adhesive role during telophase and is required for the successful completion of cytokinesis.3 It has been reported that phosphorylation–dephosphorylation cycles for Y783 and S785 are required for movement of beta1A-integrins in and out of focal contacts.4
REFERENCES
1. Schwartz, M.A. & Ginsberg, M.H.: et al: Nature Cell Biol. 4:E65-E68, 2002
2. Jin, H. et al: Br. J. Cancer 90:561-5, 2004
3. Reverte, C.G. et al: J. Cell Biol. 174:491-7, 2006
4. Sakai, T. et al: Proc. Natl. Acad. Sci. USA 98:3808-13, 2001
2. Jin, H. et al: Br. J. Cancer 90:561-5, 2004
3. Reverte, C.G. et al: J. Cell Biol. 174:491-7, 2006
4. Sakai, T. et al: Proc. Natl. Acad. Sci. USA 98:3808-13, 2001
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1251 |
Antigen: | Synthesized peptide derived from human Integrin β1 |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Rat, Mouse |
Applications & Suggested starting dilutions:* | WB 1:500-1:1000 IP n/d IHC n/d ICC n/d FACS n/d ELISA 1:1000 |
Predicted Molecular Weight of protein: | 88 kDa |
Specificity/Sensitivity: | Detects endogenous Integrin-β1 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой