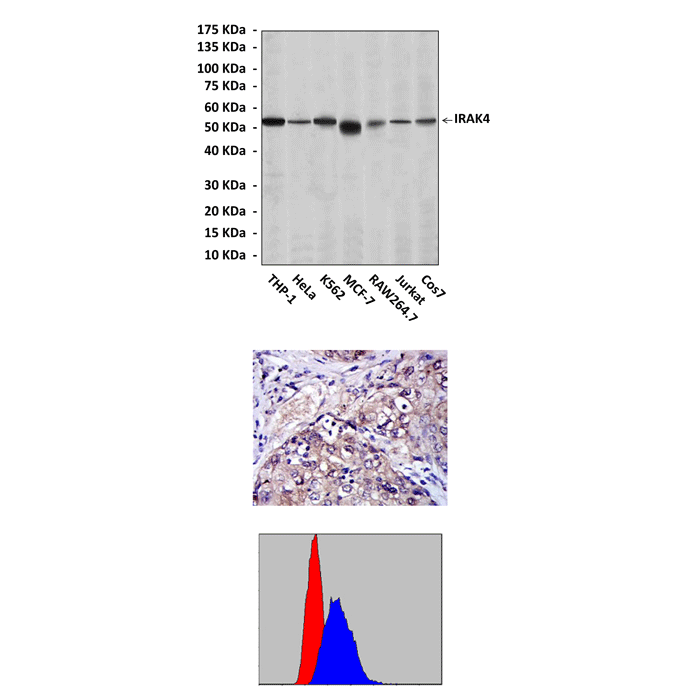
Anti-IRAK4: Mouse IRAK4 Antibody |
 |
BACKGROUND IL-1 receptor-associated kinases (IRAKs) are important mediators in the signal transduction of Toll-IL-1 receptor (TIR) family members. All TIRs with known function are involved in host defense mechanisms, either by the recognition of pathogens or as receptors for proinflammatory cytokines. They play a crucial role in the switch from innate to adaptive immunity in mammals, and the signaling cascades initiated by these receptors are implicated in a number of human diseases. The early signal transduction events triggered by the first step, the ligand-induced formation of a receptor complex. This complex can consist of a receptor and a coreceptor, such as IL-1RI and IL-1RAcP. Alternatively, several chains of one family member form a complex, as was reported for TLR4. In either case, the close proximity of the TIR domains of the individual receptor chains allows a homotypic interaction with the TIR domain of the adaptor molecules MyD88 or Mal. MyD88 can then in turn recruit an IRAK via a death domain–death domain interaction.1
There are four IRAKs: IRAK1, -2, -3(-M), and -4. IRAK-1 is activated at the receptor complex, becomes rapidly phosphorylated, and leaves the receptor complex to interact with the adaptor TRAF6. The IRAK-TRAF6 interaction is a key step in the assembly of a multiprotein signalosome, which includes the mitogen-activated protein kinase kinase kinase (MAPKKK) TAK1. This complex activates a number of downstream signaling cascades, including IkB kinases (IKKs), p38, and Jun-N-terminal kinases (JNKs), leading to the activation of transcription factors such as NF-kB and AP-1. Surprisingly, mice deficient in IRAK-1 are severely compromised in their ability to respond to IL-1, but the response is not completely abolished. This observation was explained by redundancy in the IRAK family: Two additional members IRAK-2 and IRAK-3(IRAK-M).2 Both proteins behave much like IRAK-1 in overexpression studies and they can compensate for the loss of IRAK-1 in a mutant 293 cell line. The most striking difference among the three proteins is that only IRAK-1 has potent kinase activity.3 However, IRAK-3 works as a negative Regulator of TLR/IL-1R Signaling in normal cells. IRAK-3 enhances the binding of MyD88 to IRAK-1 and IRAK-4 and prevents IRAK-1 phosphorylation.4 In this way, IRAK-M traps both IRAK molecules in the receptor complex, preventing their dissociation. IRAK-4, a novel member of the IRAK family with unique functional properties. IRAK-4 shares the domain structure of the other IRAKs and it is able to activate similar signal transduction pathways, namely NF-kB and MAPK pathways. It rapidly and transiently associates with IRAK-1 and TRAF6 in an IL-1-dependent manner but it is not functionally redundant with IRAK-1. Most strikingly, IRAK-4 is an active protein kinase and requires its kinase activity to activate NF-kB. IRAK-4 might act upstream of IRAK-1 as an IRAK-1 activator.5
There are four IRAKs: IRAK1, -2, -3(-M), and -4. IRAK-1 is activated at the receptor complex, becomes rapidly phosphorylated, and leaves the receptor complex to interact with the adaptor TRAF6. The IRAK-TRAF6 interaction is a key step in the assembly of a multiprotein signalosome, which includes the mitogen-activated protein kinase kinase kinase (MAPKKK) TAK1. This complex activates a number of downstream signaling cascades, including IkB kinases (IKKs), p38, and Jun-N-terminal kinases (JNKs), leading to the activation of transcription factors such as NF-kB and AP-1. Surprisingly, mice deficient in IRAK-1 are severely compromised in their ability to respond to IL-1, but the response is not completely abolished. This observation was explained by redundancy in the IRAK family: Two additional members IRAK-2 and IRAK-3(IRAK-M).2 Both proteins behave much like IRAK-1 in overexpression studies and they can compensate for the loss of IRAK-1 in a mutant 293 cell line. The most striking difference among the three proteins is that only IRAK-1 has potent kinase activity.3 However, IRAK-3 works as a negative Regulator of TLR/IL-1R Signaling in normal cells. IRAK-3 enhances the binding of MyD88 to IRAK-1 and IRAK-4 and prevents IRAK-1 phosphorylation.4 In this way, IRAK-M traps both IRAK molecules in the receptor complex, preventing their dissociation. IRAK-4, a novel member of the IRAK family with unique functional properties. IRAK-4 shares the domain structure of the other IRAKs and it is able to activate similar signal transduction pathways, namely NF-kB and MAPK pathways. It rapidly and transiently associates with IRAK-1 and TRAF6 in an IL-1-dependent manner but it is not functionally redundant with IRAK-1. Most strikingly, IRAK-4 is an active protein kinase and requires its kinase activity to activate NF-kB. IRAK-4 might act upstream of IRAK-1 as an IRAK-1 activator.5
REFERENCES
1. Janssens, S. & Beyaert, M.: Mol. Cell 11:293-302, 2003
2. Kanakaraj, P. et al: J. Exp. Med. 187:2073–9, 1998
3. Li, X. et al: Mol. Cell. Biol. 19:4643-52, 1999
4. Kobayashi, K. et al: Cell 110:191-202, 2002
5. Li, S. et al: Proc. Natl. Acad. Sci. USA 99:5567-72, 2002
2. Kanakaraj, P. et al: J. Exp. Med. 187:2073–9, 1998
3. Li, X. et al: Mol. Cell. Biol. 19:4643-52, 1999
4. Kobayashi, K. et al: Cell 110:191-202, 2002
5. Li, S. et al: Proc. Natl. Acad. Sci. USA 99:5567-72, 2002
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CP10418 |
Antigen: | Raised against recombinant human IRAK4 fragments expressed in E. coli. |
Isotype: | Mouse IgG1 |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:50 - 1:200 ICC n/d FACS 1:50 - 1:200 |
Predicted Molecular Weight of protein: | 52 kDa |
Specificity/Sensitivity: | Detects IRAK4 proteins in various cell lysate. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой