Anti-Phospho-IR-β: Rabbit Polyclonal Insulin Receptor-beta, Phospho-Tyr1162/1163 Antibody |
 |
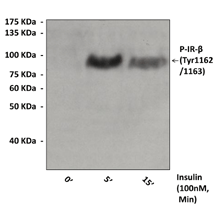
BACKGROUND Insulin Receptor is a transmembrane receptor that is activated by insulin. It belongs to the large class of tyrosine kinase receptors. Two alpha subunits and two beta subunits make up the insulin receptor. The beta subunits pass through the cellular membrane and are linked by disulfide bonds.1 Insulin receptor functions as an enzyme that transfers phosphate groups from ATP to tyrosine residues on intracellular target proteins. Binding of insulin to the alpha subunits causes the beta subunits to phosphorylate themselves (autophosphorylation), thus activating the catalytic activity of the receptor. The activation loop (A-loop) of the kinase undergoes a major conformational change upon autophosphorylation of Tyr1158, Tyr1162 and Tyr1163 within the loop, resulting in unrestricted access of ATP and protein substrates to the kinase active site. Phosphorylated Tyr1162/3 is the key phosphotyrosine in stabilizing the conformation of the tris-phosphorylated A-loop, whereas pTyr1158 is completely solvent-exposed, suggesting an availability for interaction with downstream signaling proteins. The activated receptor then phosphorylates a number of intracellular proteins, which in turn alters their activity, thereby generating a biological response.2
REFERENCES
1. Youngren, J.F.: Cell Mol Life Sci. 64:873, 2007
2. Backer, J.M. et al. J Cell Biol.118:831, 1992
2. Backer, J.M. et al. J Cell Biol.118:831, 1992
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CB4386 |
Antigen: | Synthetic peptide containing phospho-Tyr1162/3 of human insulin receptor beta sequence (1157-1170). |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions: | WB 1:1000 IP n/d IHC (Paraffin) n/d ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 97 kDa |
Specificity/Sensitivity: | Detects overexpressed levels of Tyr1162/3 phosphorylated insulin receptor-beta. This antibody does not cross-react with other ER-family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
|
| ||||||||||
| ||||||||||
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой