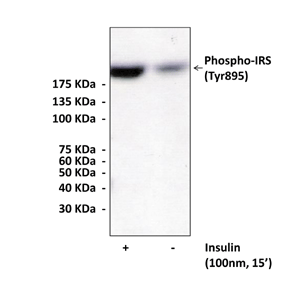
Anti-Phospho-IRS-1: Rabbit Polyclonal IRS-1, Phospho-Tyr895 Antibody |
 |
BACKGROUND Insulin Receptor Substrate (IRS) proteins play a central role in maintaining basic cellular functions such as growth and metabolism. They act as an interface between multiple growth factor receptors possessing tyrosine kinase activity, such as the insulin receptor, and a complex network of intracellular signaling molecules containing Src homology 2 (SH2) domains. Four members (IRS-1, IRS-2, IRS-3, IRS-4) of this family have been identified which differ in their subcellular distribution and interaction with SH2 domain proteins.1 Insulin receptor substrate-1 (IRS-1) is a major substrate of insulin and insulin-like growth factor-I receptors. The activated insulin receptor phosphorylates the intracellular substrate IRS-1, which then binds various signaling molecules that contain SH 2 domains, thereby propagating the insulin signal. Among these IRS-1-binding proteins, the Grb2-Sos complex and the protein tyrosine phosphatase SHP-2 transmit mitogenic signals through the activation of Ras, and phosphoinositide 3-kinase is implicated in the major metabolic actions of insulin.2 IRS-1 possesses as many as 18 potential tyrosine phosphorylation sites, several of which contain redundant motifs. Tyr895 is in a YXN motif at carboxyl terminal part of IRS-1. Phosphorylation of Tyr895 provides a binding site for Grb2, which leads to activation of downstream MAP kinase pathway.5 Insulin, also activates several kinases and these kinases act to induce the phosphorylation of IRS1 on specific serine/ threoninesites and inhibit its functions. This is part of the negative-feedback control mechanism induced by insulin that leads to termination of its action.3 Agents such as free fatty acids, cytokines, angiotensin II, endothelin-1, amino acids, cellular stress and hyperinsulinemia, which induce insulin resistance, also lead to both activation of several serine/threonine kinases and phosphorylation of IRS1. These agents negatively regulate the IRS1 functions by phosphorylation but also via others molecular mechanisms (SOCS expression, IRS degradation, O-linked glycosylation).4 IRS-1 mediates the control of various cellular processes by insulin.
REFERENCES
1. Yenush, L. & White, M.F. : Bioessays 19:491, 1997
2. Ogawa, W., Matozaki, T., & Kasuga, M. : Mol. Cell. Biochem. 182:13, 1998
3. Sesti, G. : Best Pract Res Clin Endocrinol Metab. 20:665, 2006
4. Gual, P. et al. : Biochimie 87:99, 2005
5. Sun, X. J. et al. J Biol Chem. 271:10583, 1996
2. Ogawa, W., Matozaki, T., & Kasuga, M. : Mol. Cell. Biochem. 182:13, 1998
3. Sesti, G. : Best Pract Res Clin Endocrinol Metab. 20:665, 2006
4. Gual, P. et al. : Biochimie 87:99, 2005
5. Sun, X. J. et al. J Biol Chem. 271:10583, 1996
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CB4673 |
Antigen: | Synthetic peptide containing human IRS-1 sequence (889-902) which includes phosphorylated Tyr895. |
Isotype: | Rabbit Polyclonal IgG |
Species & predicted species cross- reactivity ( ): | Human, Mouse, Rat |
Applications & Suggested starting dilutions: | WB 1:1000 IP n/d IHC (Paraffin) n/d ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 180 kDa |
Specificity/Sensitivity: | Detects endogenous levels of phosphorylated Tyr895 IRS-1 protein. This antibody is expected to recognize rat, mouse, & human Phospho-IRS. Does not cross-react with other IRS-family members |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой