Anti-KSR1: Rabbit Kinase Suppressor of Ras 1 Antibody |
 |
BACKGROUND Kinase suppressor of Ras (KSR) is a conserved component of the Ras pathway that interacts directly with MEK and MAPK. KSR constitutes a novel protein family that is related to, but distinct from, the Raf kinase family. KSR proteins are found in Drosophila, Caenorhabditis elegans, and mammals but not yeast. Members of the KSR family contain five conserved protein domains (CA1 to 5). CA1 is a 40-amino-acid domain unique to the KSR proteins, CA2 is a proline-rich domain, CA3 is a cysteine-rich domain, CA4 is a serine-threonine-rich domain, and CA5 constitutes the catalytic domain. CA1 to 4 are located in the amino-terminal region of the protein, whereas CA5 is found in the carboxy-terminal region. Surprisingly, while the KSR proteins are predicted to be protein kinases, no physiological substrates of KSR have been identified, nor has KSR been conclusively demonstrated to possess intrinsic kinase activity.1
KSR was first identified to be a positive effector of Ras signaling by genetic studies performed in Drosophila and C. elegans. Evaluating the contribution of mammalian KSR to Ras signaling, however, has been more difficult since experiments addressing KSR function in mammalian cells have yielded conflicting results. In some reports, expression of murine KSR1 enhanced the biological activity of activated Ras by accelerating the activation of MEK and MAPK. In contrast, other studies found that KSR1 expression inhibited Ras signaling by either blocking MEK and MAPK activation or inhibiting Elk-1 phosphorylation. The discrepancy in these findings appears to be due to the level of KSR protein expressed. It was demonstrated that, KSR1 functioned as a positive regulator of Ras signaling when expressed at low levels, whereas at high levels of expression, KSR1 blocked Ras-mediated signal transduction. Indeed, altering the expression level of KSR1 can modulate the actions of growth factors and oncogenes.2
KSR1 serves as a docking platform for the authentic kinase components of the Ras/MAPK cascade. KSR1 translocates from the cytoplasm to the cell surface in response to growth factor treatment and that this process is regulated by Cdc25C-associated kinase 1 (C-TAK1). C-TAK1 constitutively associates with mammalian KSR1 and phosphorylates serine 392 to confer 14-3-3 binding and cytoplasmic sequestration of KSR1 in unstimulated cells. In response to signal activation, the phosphorylation state of S392 is reduced, allowing the KSR1 complex to colocalize with activated Ras and Raf-1 at the plasma membrane, thereby facilitating the phosphorylation reactions required for the activation of MEK and MAPK.3 Protein Phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites.4
REFERENCES
1. Ritt, D.A. et al: Method Enzymol. 407:224-37, 2006
2. Kortum, R. & Lewis, R.E.: Mol. Cell. Biol. 24:4407-16, 2004
3. Muller, J. et al: Mol. Cell 8:983-93, 2001
4. Ory, S. et al: Curr. Biol. 13:1356-64, 2003
2. Kortum, R. & Lewis, R.E.: Mol. Cell. Biol. 24:4407-16, 2004
3. Muller, J. et al: Mol. Cell 8:983-93, 2001
4. Ory, S. et al: Curr. Biol. 13:1356-64, 2003
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
Cat.No.: | CG1215 |
Antigen: | Kinase suppressor of Ras 1 recombinant protein epitope signature tag (PrEST). |
Isotype: | Rabbit IgG |
Species & predicted species cross- reactivity ( ): | Human |
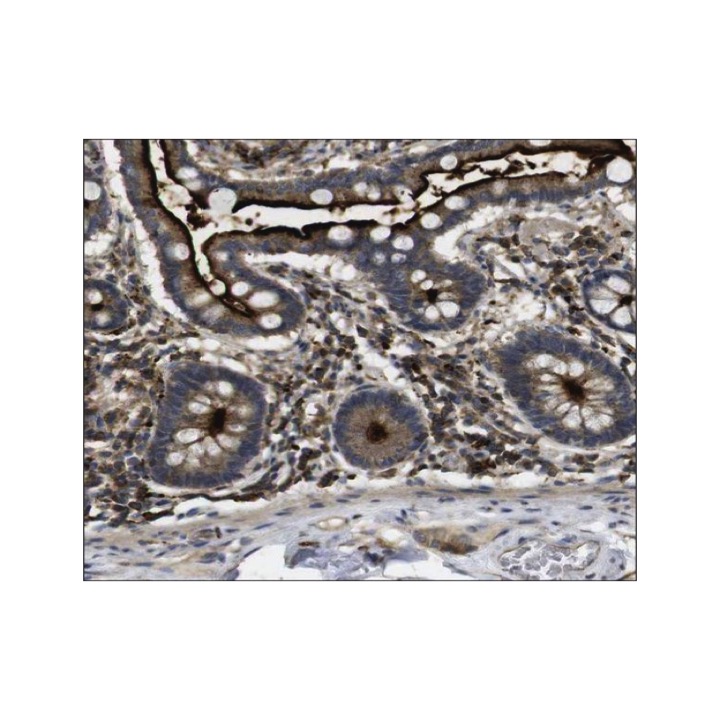
Applications & Suggested starting dilutions:* | WB n/d IP n/d IHC 1:20-1:50 ICC n/d FACS n/d |
Predicted Molecular Weight of protein: | 31 kDa |
Specificity/Sensitivity: | Detects endogenous KSR1 proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Документы
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой